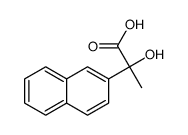
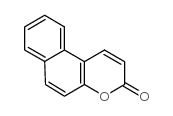
Splitomicin
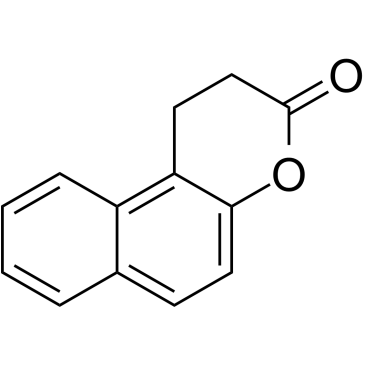
Splitomicin structure
|
Common Name | Splitomicin | ||
|---|---|---|---|---|
| CAS Number | 5690-03-9 | Molecular Weight | 198.217 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 383.6±17.0 °C at 760 mmHg | |
| Molecular Formula | C13H10O2 | Melting Point | 73-74.5℃ | |
| MSDS | Chinese USA | Flash Point | 161.2±18.4 °C | |
Use of SplitomicinSplitomicin (Splitomycin) is a selective Sir2p inhibitor. Splitomicin inhibits NAD+-dependent HDAC activity of Sir2 protein. Splitomicin induces dose-dependent inhibition of HDAC in the yeast extract with an IC50 of 60 μM[1]. |
| Name | Splitomicin |
|---|---|
| Synonym | More Synonyms |
| Description | Splitomicin (Splitomycin) is a selective Sir2p inhibitor. Splitomicin inhibits NAD+-dependent HDAC activity of Sir2 protein. Splitomicin induces dose-dependent inhibition of HDAC in the yeast extract with an IC50 of 60 μM[1]. |
|---|---|
| Related Catalog | |
| Target |
Sir2p:60 μM (IC50) |
| In Vivo | Splitomicin (80 mg/kg with an intraperitoneal injection every 24 h for 5 days, in mice) enhances tissue factor (TF) activity in the arterial vessel wall and accelerates carotid artery thrombus formation in a photochemical injury model[2]. Animal Model: C57BL/6 mice aged 12-14 weeks weighing on average 27 g[2] Dosage: 80 mg/kg Administration: Intraperitoneal injection every 24 h for 5 days Result: Increased TF activity in mouse carotid artery as compared with the controls. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 383.6±17.0 °C at 760 mmHg |
| Melting Point | 73-74.5℃ |
| Molecular Formula | C13H10O2 |
| Molecular Weight | 198.217 |
| Flash Point | 161.2±18.4 °C |
| Exact Mass | 198.068085 |
| PSA | 26.30000 |
| LogP | 3.03 |
| Appearance of Characters | solid |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.656 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914399090 |
|
~87% 
Splitomicin CAS#:5690-03-9 |
| Literature: National University Corporation Nagoya University; ISHIHARA, Kazuaki; UYANIK, Muhammet; YASUI, Takeshi Patent: EP2684863 A1, 2014 ; Location in patent: Paragraph 0106 ; |
|
~% 
Splitomicin CAS#:5690-03-9 |
| Literature: Journal of the American Chemical Society, , vol. 70, p. 2119 |
|
~% 
Splitomicin CAS#:5690-03-9 |
| Literature: Journal of the American Chemical Society, , vol. 70, p. 2119 |
|
~% 
Splitomicin CAS#:5690-03-9 |
| Literature: Journal of Chemical Research, Miniprint, , # 11 p. 1175 - 1184 |
|
~% 
Splitomicin CAS#:5690-03-9 |
| Literature: Journal of Organic Chemistry, , vol. 20, p. 1282,1285 |
|
~% 
Splitomicin CAS#:5690-03-9 |
| Literature: Angewandte Chemie - International Edition, , vol. 50, # 26 p. 5834 - 5838 |
|
~% 
Splitomicin CAS#:5690-03-9 |
| Literature: US2005/79995 A1, ; Page/Page column 13 ; |
|
~% 
Splitomicin CAS#:5690-03-9 |
| Literature: Sb. Statei Obshch. Khim., , p. 610,613 Chem.Abstr., , p. 986 Journal of the American Chemical Society, , vol. 70, p. 2119 |
| HS Code | 2914399090 |
|---|---|
| Summary | 2914399090. other aromatic ketones without other oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis.
Eur. Heart J. 31 , 2301-9, (2010) Endothelial activation, macrophage infiltration, and foam cell formation are pivotal steps in atherogenesis. Our aim in this study was to analyse the role of SIRT1, a class III deacetylase with import... |
|
|
Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection.
PLoS ONE 4(6) , e6093, (2009) The persistence of transcriptionally silent but replication-competent HIV-1 reservoirs in Highly Active Anti-Retroviral Therapy (HAART)-treated infected individuals, represents a major hurdle to virus... |
|
|
Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells.
Cardiovasc. Res. 89 , 464-72, (2011) The mammalian silent information regulator-two 1 (Sirt1) blunts the noxious effects of cardiovascular risk factors such as type 2 diabetes mellitus and obesity. Nevertheless, the role of Sirt1 in regu... |
| Splitomicin |
| 1,2-dihydrobenzo[f]chromen-3-one |
| 1,2-Dihydro-3H-benzo[f]chromen-3-one |
| MFCD03792600 |
| 3H-Naphtho[2,1-b]pyran-3-one, 1,2-dihydro- |

![3,4-dihydro-2H-naphtho[2,3-b]pyran-2-one structure](https://image.chemsrc.com/caspic/341/759458-82-7.png)
![ethyl 3-oxo-2,3-dihydro-1H-benzo[f]chromene-2-carboxylate structure](https://image.chemsrc.com/caspic/175/14103-18-5.png)