Nosiheptide
Modify Date: 2025-08-20 13:55:28
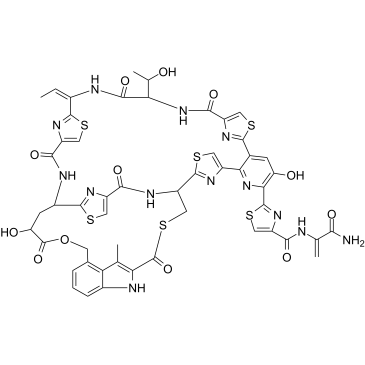
Nosiheptide structure
|
Common Name | Nosiheptide | ||
|---|---|---|---|---|
| CAS Number | 56377-79-8 | Molecular Weight | 1222.357 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C51H43N13O12S6 | Melting Point | 310-320° (dec) | |
| MSDS | N/A | Flash Point | N/A | |
Use of NosiheptideNosiheptide (Multhiomycin), a thiopeptide antibiotic produced by Streptomyces actuosus, inhibits bacterial protein synthesis and bears a unique indole side ring system and regiospecific hydroxyl groups on the characteristic macrocyclic core. Nosiheptide has been widely used as a feed additive for animal growth[1][2]. |
| Name | Nosiheptide |
|---|---|
| Synonym | More Synonyms |
| Description | Nosiheptide (Multhiomycin), a thiopeptide antibiotic produced by Streptomyces actuosus, inhibits bacterial protein synthesis and bears a unique indole side ring system and regiospecific hydroxyl groups on the characteristic macrocyclic core. Nosiheptide has been widely used as a feed additive for animal growth[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Nosiheptide exhibits extremely potent activity against all contemporary Staphylococcus aureus strains tested including multiple drug-resistant clinical isolates, with MIC values ≤ 0.25 mg/L. Nosiheptide is also highly active against Enterococcus spp and the contemporary hypervirulent BI strain of Clostridium difficile but is inactive against most Gram-negative strains tested. Time-kill analysis reveals Nosiheptide to be rapidly bactericidal against Staphylococcus aureus in a concentration- and time-dependent manner, with a nearly 2-log kill noted at 6 hours at 10X MIC. Furthermore, Nosiheptide is found to be non-cytotoxic against mammalian cells at >> 100X MIC, and its anti-Staphylococcus aureus activity is not inhibited by 20% human serum. Notably, Nosiheptide exhibits a significantly prolonged post-antibiotic effect against both healthcare- and community-associated Staphylococcus aureus compared to vancomycin[1]. |
| In Vivo | Nosiheptide (20 mg/kg; intraperitoneal injection; injected at 1 and 8 h post-infection; female CD1 mice) provids significant protection against mortality. Ten out of 10 of the Nosiheptide-treated mice remains alive on day 3, while 6/10 of the controls died on day 1[1]. Animal Model: Eight week old female CD1 mice injected with HA-Staphylococcus aureus strain Sanger 252[1] Dosage: 20 mg/kg Administration: Intraperitoneal injection; injected at 1 and 8 h post-infection Result: Provided significant protection against mortality. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Melting Point | 310-320° (dec) |
| Molecular Formula | C51H43N13O12S6 |
| Molecular Weight | 1222.357 |
| Exact Mass | 1221.147827 |
| PSA | 552.28000 |
| LogP | 0.72 |
| Index of Refraction | 1.699 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 2-[(1S,7S,14S,16S)-10-(Z)-ethylidene-35,16-dihydroxy-7-((R)-1-hydroxy-ethyl)-203-methyl-5,8,12,17,21,25-hexaoxo-201H-18-oxa-22-thia-6,9,13,24-tetraaza-20(4,2)-indola-3(2,3)-pyridina-2,4,11,26(2,4)-tetrakis-thiazola-bicyclo[12.9.3]hexacosaphan-36-yl]-thiazole-4-carboxylic acid 1-carbamoyl-vinylamide |
| Multhiomycin |
| Nosiheptide |
| priMofax |
| N-[1-(Aminocarbonyl)ethenyl]-2-[(11S,14Z,21S,23S,29S)-14-ethylidene-9,10,11,12,13,14,19,20,21,22,23,24,26,33,35,36-hexadecahydro-3,23-dihydroxy-11-[(1R)-1-hydroxyethyl]-31-methyl-9,12,19,24,33,43-hexaoxo-30,32-imino-8,5:18,15:40,37-trinitrilo-21,36-([2,4]-endo-thiazolomethanimino)-5H,15H,37H-pyrido[3,2-w][2,11,21,27,31,7,14,17]benzoxatetrathiatriazacyclohexatriacontin-2-yl]-4-thiazolecarboxamide |
| N-(3-Amino-3-oxoprop-1-en-2-yl)-2-[(21Z)-21-ethylidene-9,30-dihydroxy-18-(1-hydroxyethyl)-40-methyl-16,19,26,31,42,46-hexaoxo-32-oxa-3,13,23,43,49-pentathia-7,17,20,27,45,51,52,53,54,55-decaazanonacyclo[26.16.6.1.1.1.1.1.0.0]pentapentaconta-2(55),4,6,8,10,12(54),14,22(53),24,34,36,38,40,47,50-pentadecaen-8-yl]-1,3-thiazole-4-carboxamide |
| N-(3-amino-3-oxoprop-1-en-2-yl)-2-[(21Z)-21-ethylidene-9,30-dihydroxy-18-(1-hydroxyethyl)-40-methyl-16,19,26,31,42,46-hexaoxo-32-oxa-3,13,23,43,49-pentathia-7,17,20,27,45,51,52,53,54,55-decaazanonacyclo[26.16.6.1.1.1.1.1.0.0]pentapentaconta-2(55),4,6,8,10,12(54),14,22(53),24,34,36,38,40,47,50-pentadecaen-8-yl]-1,3-thiazole-4-carboxamide (non-preferred name) |
| N-(3-Amino-3-oxo-1-propen-2-yl)-2-[(21Z)-21-ethylidene-9,30-dihydroxy-18-(1-hydroxyethyl)-40-methyl-16,19,26,31,42,46-hexaoxo-32-oxa-3,13,23,43,49-pentathia-7,17,20,27,45,51,52,53,54,55-decaazanonacyc ;lo[26.16.6.1.1.1.1.1.0.0]pentapentaconta-2(55),4,6,8,10,12(54),14,22(53),24,34,36,38,40,47,50-pentadecaen-8-yl]-1,3-thiazole-4-carboxamide |