Furamidine dihydrochloride
Modify Date: 2025-08-22 17:07:50
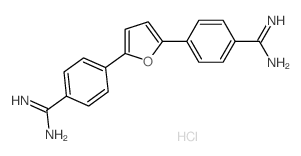
Furamidine dihydrochloride structure
|
Common Name | Furamidine dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 55368-40-6 | Molecular Weight | 340.80700 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C18H17ClN4O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Furamidine dihydrochlorideFuramidine dihydrochloride (DB75 dihydrochloride) is a selective protein arginine methyltransferase 1 (PRMT1) inhibitor with an IC50 of 9.4 μM. Furamidine dihydrochloride is selective for PRMT1 over PRMT5, PRMT6, and PRMT4 (CARM1) (IC50s of 166 µM, 283 µM, and >400 µM, respectively). Furamidine dihydrochloride is a potent, reversible and competitive tyrosyl-DNA phosphodiesterase 1 (TDP-1) inhibitor. Inhibition of TDP-1 by Furamidine dihydrochloride is effective both with single- and double-stranded DNA substrates but is slightly stronger with the duplex DNA. Furamidine dihydrochloride is also an antiparasite agent[1][2][3]. |
| Name | Benzenecarboximidamide, 4,4'-(2,5-furandiyl)bis-, dihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Furamidine dihydrochloride (DB75 dihydrochloride) is a selective protein arginine methyltransferase 1 (PRMT1) inhibitor with an IC50 of 9.4 μM. Furamidine dihydrochloride is selective for PRMT1 over PRMT5, PRMT6, and PRMT4 (CARM1) (IC50s of 166 µM, 283 µM, and >400 µM, respectively). Furamidine dihydrochloride is a potent, reversible and competitive tyrosyl-DNA phosphodiesterase 1 (TDP-1) inhibitor. Inhibition of TDP-1 by Furamidine dihydrochloride is effective both with single- and double-stranded DNA substrates but is slightly stronger with the duplex DNA. Furamidine dihydrochloride is also an antiparasite agent[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 9.4 μM (Protein arginine methyltransferase 1 (PRMT1)); 166 µM (PRMT5), 283 µM (PRMT6) and >400 µM (PRMT4)[1] Parasite[1] Tyrosyl-DNA phosphodiesterase 1 (TDP-1)[2] |
| In Vitro | Furamidine (compound 1; 20 μM; 72 hours; leukemia cell lines) inhibits cell growth for most of the leukemia cell lines except HEL cells which have JAK2V617F mutations[1]. Furamidine (compound 1; 20 μM; 15 hours; 293T cells) treatment significantly reduces the expression level of the methylated GFP-ALY protein in 293T cells[1]. Furamidine binds duplex DNA in the DNA minor groove selectively at AT rich sites [(A/T)4]. Furamidine can also intercalate between GC base pairs of duplex DNA. Furamidine could therefore interfere with DNA processing enzymes such as TDP-1[2]. Cell Viability Assay[1] Cell Line: Meg-01, K562, HL-60, NB4, MOLM13, HEL, CMK, CMY, CMS and CHRF cells Concentration: 20 μM Incubation Time: 72 hours Result: Inhibited cell growth for most of the leukemia cell lines except HEL cells which have JAK2V617F mutations. Western Blot Analysis[1] Cell Line: 293T cells Concentration: 20 μM Incubation Time: 15 hours Result: The expression level of the methylated GFP-ALY protein is significantly reduced. |
| In Vivo | Furamidine (1 mg/kg; intraperitoneal injection; 3 times a week and repeated every 4 weeks; for 34 weeks; female NZB/NZW mice) and Irinotecan combined treatment suppresses proteinuria and prolongs survival of lupus-prone NZB/NZW mice. The combination treatment does not change the levels of anti-dsDNA antibodies[3]. Animal Model: Female NZB/NZW mice (6-week-old) with Irinotecan (1 mg/kg)[3] Dosage: 1 mg/kg Administration: Intraperitoneal injection; 3 times a week and repeated every 4 weeks; for 34 weeks Result: Suppressed proteinuria and prolongs survival of lupus-prone NZB/NZW mice combined with Irinotecan. |
| References |
| Molecular Formula | C18H17ClN4O |
|---|---|
| Molecular Weight | 340.80700 |
| Exact Mass | 340.10900 |
| PSA | 112.88000 |
| LogP | 5.58380 |
| InChIKey | OPUODUNVVMCWGH-UHFFFAOYSA-N |
| SMILES | Cl.N=C(N)c1ccc(-c2ccc(-c3ccc(C(=N)N)cc3)o2)cc1 |
| Storage condition | -20°C |
| Furamidine |
| DB75 |
| FuraMidine Dihydrochloride |