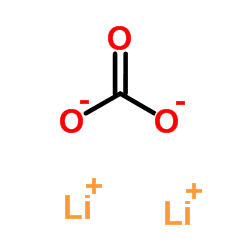
Dilithium oxalate
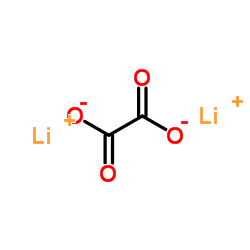
Dilithium oxalate structure
|
Common Name | Dilithium oxalate | ||
|---|---|---|---|---|
| CAS Number | 553-91-3 | Molecular Weight | 101.901 | |
| Density | 0.99 g/mL±0.002 g/mL at 20 °C | Boiling Point | 100 °C(lit.) | |
| Molecular Formula | C2Li2O4 | Melting Point | 41-45 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | Lithium Oxalate |
|---|---|
| Synonym | More Synonyms |
| Density | 0.99 g/mL±0.002 g/mL at 20 °C |
|---|---|
| Boiling Point | 100 °C(lit.) |
| Melting Point | 41-45 °C(lit.) |
| Molecular Formula | C2Li2O4 |
| Molecular Weight | 101.901 |
| Flash Point | >230 °F |
| Exact Mass | 102.011665 |
| PSA | 80.26000 |
| Index of Refraction | n20/D 1.461 |
| InChIKey | YNQRWVCLAIUHHI-UHFFFAOYSA-L |
| SMILES | O=C([O-])C(=O)[O-].[Li+].[Li+] |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
|
Section 1. Chemical Product and Company Identification Lithium OxalateCatalog Common Name/ Number(s). Trade Name CAS#553-91-3 Manufacturer
RTECSNot available. SPECTRUM CHEMICAL MFG. CORP. TSCATSCA 8(b) inventory: No products were found. Commercial Name(s)Not available. CI# Not available. SynonymNot available. IN CASE OF EMERGENCY Lithium Oxalate Chemical Name Chemical FamilyNot available.CALL (310) 516-8000 C2-Li2-O4 Chemical Formula SPECTRUM CHEMICAL MFG. CORP. Section 2.Composition and Information on Ingredients Exposure Limits TWA (mg/m3)STEL (mg/m3) CEIL (mg/m3) NameCAS #% by Weight 1) Lithium Oxalate553-91-3100 Toxicological DataLithium Oxalate on IngredientsLD50: Not available. LC50: Not available. Section 3. Hazards Identification Potential Acute Health Effects Hazardous in case of eye contact (irritant), of ingestion. Potential Chronic HealthCARCINOGENIC EFFECTS: Not available. EffectsMUTAGENIC EFFECTS: Not available. TERATOGENIC EFFECTS: Not available. DEVELOPMENTAL TOXICITY: Not available. Repeated or prolonged exposure is not known to aggravate medical condition. Lithium Oxalate Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. Immediately flush eyes with running water for at least 15 minutes, keeping eyelids open. Cold water may be used. Do not use an eye ointment. Seek medical attention. Skin ContactNo known effect on skin contact, rinse with water for a few minutes. Serious Skin ContactNot available. InhalationAllow the victim to rest in a well ventilated area. Seek immediate medical attention. Serious InhalationNot available. IngestionDo not induce vomiting. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material was ingested; the absence of such signs, however, is not conclusive. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth resuscitation. Seek immediate medical attention. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Not available. Flash Points Not available. Flammable Limits Products of CombustionSome metallic oxides. Fire Hazards in Presence of Not available. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. of Various SubstancesRisks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaSMALL FIRE: Use DRY chemical powder. and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. Not available. Special Remarks on Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Lithium Oxalate Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the residue under a fume hood. Ground all equipment containing material. Do not ingest. Do not breathe dust. Avoid contact with eyes Wear suitable protective clothing If ingested, seek medical advice immediately and show the container or the label. Keep away from incompatibles such as oxidizing agents. StorageKeep container dry. Keep in a cool place. Ground all equipment containing material. Keep container tightly closed. Keep in a cool, well-ventilated place. Combustible materials should be stored away from extreme heat and away from strong oxidizing agents. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSplash goggles. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Crystalline solid.)OdorNot available. TasteNot available. 101.96 g/mole Molecular Weight ColorColorless. pH (1% soln/water)Not available. Boiling PointNot available. Melting PointNot available. Critical TemperatureNot available. Specific GravityNot available. Not applicable. Vapor Pressure Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Not available. Ionicity (in Water) Dispersion PropertiesVery slightly dispersed in cold water, hot water. Very slightly soluble in cold water, hot water. Solubility Section 10. Stability and Reactivity Data The product is stable. Stability Instability TemperatureNot available. Not available. Conditions of Instability Incompatibility with various Reactive with oxidizing agents. substances Lithium Oxalate CorrosivityNot available. Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity PolymerizationNo. Section 11. Toxicological Information Routes of EntryEye contact. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans Not available. Other Toxic Effects onHazardous in case of ingestion. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onNot available. Chronic Effects on Humans Special Remarks on otherNot available. Toxic Effects on Humans Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. Toxicity of the ProductsThe products of degradation are less toxic than the product itself. of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste Disposal Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). IdentificationNot applicable. Special Provisions forNot applicable. Transport Lithium Oxalate DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms No products were found. Federal and State Regulations California Proposition 65 Warnings Other RegulationsNot available.. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC)R20/21/22- Harmful by inhalation, in contact with skin and if swallowed. Health Hazard HMIS (U.S.A.)2 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 2 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Lithium Oxalate SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R21/22 |
| Safety Phrases | S26-S27-S36/37/39-S45 |
| RIDADR | UN 1759 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2917119000 |
|
~% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Chemistry Letters, , # 11 p. 2175 - 2178 |
|
~% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Tetrahedron Letters, , vol. 39, # 45 p. 8275 - 8276 |
|
~% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Tetrahedron Letters, , vol. 39, # 45 p. 8275 - 8276 |
|
~% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Tetrahedron Letters, , vol. 39, # 45 p. 8275 - 8276 |
|
~% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Justus Liebigs Annalen der Chemie, vol. 146, p. 140 |
|
~% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Annalen der Physik (Weinheim, Germany), , vol. 62, p. 399 - 399 Ann. Chim. Phys., , vol. 51, p. 140 - 140 |
|
~% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Liebigs Annalen der Chemie, , vol. 100, p. 310 - 310 Ann. Chim. Phys., , vol. 51, p. 142 - 142 Handbuch der krystallographisch-physikalischen Chemie, Bd. 2, Leipzig 1882, S. 50 |
|
~0% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Gorski, A.; Krasnicka, A. D. Journal of Thermal Analysis, 1987 , vol. 32, p. 1895 - 1904 |
|
~0% 
Dilithium oxalate CAS#:553-91-3 |
| Literature: Gorski, A.; Krasnicka, A. D. Journal of Thermal Analysis, 1987 , vol. 32, p. 1895 - 1904 |
| HS Code | 2917119000 |
|---|---|
| Summary | 2917119000 oxalic acid salts and esters VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Are fluoride-containing blood tubes still needed for glucose testing?
Clin. Biochem. 46(4-5) , 289-90, (2013)
|
|
|
Isolation and characterization of oxalotrophic bacteria from tropical soils.
Arch. Microbiol. 197(1) , 65-77, (2015) The oxalate-carbonate pathway (OCP) is a biogeochemical set of reactions that involves the conversion of atmospheric CO2 fixed by plants into biomass and, after the biological recycling of calcium oxa... |
|
|
Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements.
Environ. Sci. Pollut. Res. Int. 20(3) , 1876-80, (2013) The presence of Eriophorum angustifolium in mine tailings of pyrite maintains a neutral pH, despite weathering, thus lowering the release of toxic elements into acid mine drainage water. We investigat... |
| lithium oxalate |
| Oxalic acid, dilithium salt |
| Ethanedioic acid, lithium salt (1:2) |
| EINECS 209-054-1 |
| Dilithium oxalate |
| MFCD00040596 |
| Ethanedioic acid, dilithium salt |






 CAS#:7440-44-0
CAS#:7440-44-0
