2-Bromothiophene-5-Sulfonamide
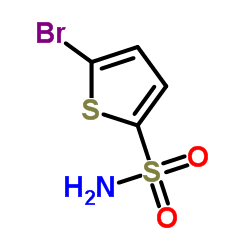
2-Bromothiophene-5-Sulfonamide structure
|
Common Name | 2-Bromothiophene-5-Sulfonamide | ||
|---|---|---|---|---|
| CAS Number | 53595-65-6 | Molecular Weight | 242.114 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | 386.4±52.0 °C at 760 mmHg | |
| Molecular Formula | C4H4BrNO2S2 | Melting Point | 138-142 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 187.5±30.7 °C | |
| Name | 5-Bromothiophene-2-sulfonamide |
|---|---|
| Synonym | More Synonyms |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 386.4±52.0 °C at 760 mmHg |
| Melting Point | 138-142 °C(lit.) |
| Molecular Formula | C4H4BrNO2S2 |
| Molecular Weight | 242.114 |
| Flash Point | 187.5±30.7 °C |
| Exact Mass | 240.886673 |
| PSA | 96.78000 |
| LogP | 1.03 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.647 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2935009090 |
|
~98% 
2-Bromothiophen... CAS#:53595-65-6 |
| Literature: Yates, Matthew H.; Kallman, Neil J.; Ley, Christopher P.; Wei, Jeffrey N. Organic Process Research and Development, 2009 , vol. 13, # 2 p. 255 - 262 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Synthesis, Density Functional Theory (DFT), Urease Inhibition and Antimicrobial Activities of 5-Aryl Thiophenes Bearing Sulphonylacetamide Moieties.
Molecules 20 , 19914-28, (2015) A variety of novel 5-aryl thiophenes 4a-g containing sulphonylacetamide (sulfacetamide) groups were synthesized in appreciable yields via Pd[0] Suzuki cross coupling reactions. The structures of these... |
|
|
Cerebrovasodilatation through selective inhibition of the enzyme carbonic anhydrase. 3. 5-(Arylthio)-, 5-(arylsulfinyl)-, and 5-(arylsulfonyl)thiophene-2-sulfonamides.
J. Med. Chem. 24(8) , 959-64, (1981) A series of 5-(arylthio)-, 5-(arylsulfinyl)-, and 5-(arylsulfonyl)thiophene-2-sulfonamides is described and anticonvulsant activities are listed for the compounds. In most cases, the sulfones had the ... |
|
|
Acyl sulfonamide anti-proliferatives. Part 2: activity of heterocyclic sulfonamide derivatives.
Bioorg. Med. Chem. Lett. 15(3) , 617-20, (2005) The anti-proliferative activity of acylated heterocyclic sulfonamides is described in Vascular Endothelial Growth Factor-dependent Human Umbilical Vascular Endothelial Cells (VEGF-HUVEC) and in HCT116... |
| 5-Bromothiophene-2-sulfonamide |
| MFCD00067990 |
| 5-Bromo-thiophene-2-sulfonic acid amide |
| 2-Thiophenesulfonamide, 5-bromo- |
| 5-Bromo-2-thiophenesulfonamide |
| 2-Bromothiophene-5-Sulfonamide |
| 2-bromo thiophene-5-sulfonamide |
 CAS#:63031-79-8
CAS#:63031-79-8 CAS#:519055-62-0
CAS#:519055-62-0![5-[(4-CHLOROPHENYL)THIO]THIOPHENE-2-SULFONAMIDE structure](https://www.chemsrc.com/caspic/326/63031-81-2.png) CAS#:63031-81-2
CAS#:63031-81-2
