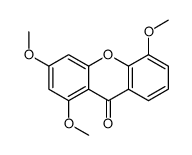
1,3,5-Trihydroxy-4-prenylxanthone

1,3,5-Trihydroxy-4-prenylxanthone structure
|
Common Name | 1,3,5-Trihydroxy-4-prenylxanthone | ||
|---|---|---|---|---|
| CAS Number | 53377-61-0 | Molecular Weight | 312.317 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 550.7±49.0 °C at 760 mmHg | |
| Molecular Formula | C18H16O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 204.3±23.3 °C | |
Use of 1,3,5-Trihydroxy-4-prenylxanthone1,3,5-Trihydroxy-4-prenylxanthone is a Na+/H+ exchange system (Na+/H+ Exchanger (NHE)) inhibitor with a minimum inhibitory concentration of 10 μg/mL[1]. 1,3,5-Trihydroxy-4-prenylxanthone is a phosphodiesterase type 5 (PDE5) (Phosphodiesterase (PDE)) inhibitor with an IC50 value of 3.0 μM[3]. 1,3,5-Trihydroxy-4-prenylxanthone inhibits Lipopolysaccharide (LPS) (Lipopolysaccharides (HY-D1056))-induced NO production in RAW264.7 macrophages, and has anti-inflammatory activities[2]. |
| Name | 1,3,5-Trihydroxy-4-(3-methyl-2-buten-1-yl)-9H-xanthen-9-one |
|---|---|
| Synonym | More Synonyms |
| Description | 1,3,5-Trihydroxy-4-prenylxanthone is a Na+/H+ exchange system (Na+/H+ Exchanger (NHE)) inhibitor with a minimum inhibitory concentration of 10 μg/mL[1]. 1,3,5-Trihydroxy-4-prenylxanthone is a phosphodiesterase type 5 (PDE5) (Phosphodiesterase (PDE)) inhibitor with an IC50 value of 3.0 μM[3]. 1,3,5-Trihydroxy-4-prenylxanthone inhibits Lipopolysaccharide (LPS) (Lipopolysaccharides (HY-D1056))-induced NO production in RAW264.7 macrophages, and has anti-inflammatory activities[2]. |
|---|---|
| Related Catalog | |
| Target |
PDE5:3 μM (IC50) iNOS Na+/H+ Exchanger |
| In Vitro | 1,3,5-Trihydroxy-4-prenylxanthone(10-30 μM;18 小时)通过消除 IKK 磷酸化、IκB 降解和 NF-κB 核转位抑制 LPS 诱导的 iNOS 表达。 1,3,5-Trihydroxy-4-prenylxanthone 通过干扰 IRAK-1 的翻译后修饰来抑制 LPS 诱导的 iNOS 表达,从而阻断 TAK1 介导的 IKK 和 MAPK 信号转导激活,从而下调 NF-κB 和 AP-1 的激活[2]。 Western Blot Analysis[2] Cell Line: RAW264.7 macrophages Concentration: 10 μM, 20 μM and 30 μM Incubation Time: 18 hours Result: Showed suppression of LPS-induced iNOS expression. Suppressed the nuclear level of c-Fos and c-Jun (major components of activator protein-1, AP-1) and the phosphorylated level of upstream signal molecules, such as JNK and ERK. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 550.7±49.0 °C at 760 mmHg |
| Molecular Formula | C18H16O5 |
| Molecular Weight | 312.317 |
| Flash Point | 204.3±23.3 °C |
| Exact Mass | 312.099762 |
| PSA | 90.90000 |
| LogP | 3.26 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.676 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2932999099 |
|
~% 
1,3,5-Trihydrox... CAS#:53377-61-0 |
| Literature: Helesbeux, Jean-Jacques; Duval, Olivier; Dartiguelongue, Caroline; Seraphin, Denis; Oger, Jean-Michel; Richomme, Pascal Tetrahedron, 2004 , vol. 60, # 10 p. 2293 - 2300 |
|
~% 
1,3,5-Trihydrox... CAS#:53377-61-0 |
| Literature: Helesbeux, Jean-Jacques; Duval, Olivier; Dartiguelongue, Caroline; Seraphin, Denis; Oger, Jean-Michel; Richomme, Pascal Tetrahedron, 2004 , vol. 60, # 10 p. 2293 - 2300 |
|
~% 
1,3,5-Trihydrox... CAS#:53377-61-0 |
| Literature: Helesbeux, Jean-Jacques; Duval, Olivier; Dartiguelongue, Caroline; Seraphin, Denis; Oger, Jean-Michel; Richomme, Pascal Tetrahedron, 2004 , vol. 60, # 10 p. 2293 - 2300 |
|
~% 
1,3,5-Trihydrox... CAS#:53377-61-0 |
| Literature: Helesbeux, Jean-Jacques; Duval, Olivier; Dartiguelongue, Caroline; Seraphin, Denis; Oger, Jean-Michel; Richomme, Pascal Tetrahedron, 2004 , vol. 60, # 10 p. 2293 - 2300 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 9H-Xanthen-9-one, 1,3,5-trihydroxy-4-(3-methyl-2-buten-1-yl)- |
| 1,3,5-trihydroxy-4-(3-methylbut-2-enyl)xanthen-9-one |
| 1,3,5-Trihydroxy-4-(3-methyl-2-buten-1-yl)-9H-xanthen-9-one |
| 2,4,6-trihydroxynitrobenzene |
| nitrophlorogucinol |
| Nitrophloroglucinol |
| 1-Nitrophloroglucinol |
| 1,3,5-trihydroxy-4-(3-methylbut-2-enyl)-9H-xanthen-9-one |
| 1,3,5-Trihydroxy-2-nitrobenzene |
| 2-nitro-phloroglucinol |
| hitrophloroglucinol |