4-Aminoindole

4-Aminoindole structure
|
Common Name | 4-Aminoindole | ||
|---|---|---|---|---|
| CAS Number | 5192-23-4 | Molecular Weight | 132.163 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 354.0±15.0 °C at 760 mmHg | |
| Molecular Formula | C8H8N2 | Melting Point | 106-109 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 195.0±7.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1H-indol-4-amine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 354.0±15.0 °C at 760 mmHg |
| Melting Point | 106-109 °C(lit.) |
| Molecular Formula | C8H8N2 |
| Molecular Weight | 132.163 |
| Flash Point | 195.0±7.6 °C |
| Exact Mass | 132.068741 |
| PSA | 41.81000 |
| LogP | 0.86 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.758 |
| Storage condition | −20°C |
| Water Solubility | Insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29339990 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis and in vitro and in vivo antitumor activity of 2-phenylpyrroloquinolin-4-ones.
J. Med. Chem. 48(9) , 3417-27, (2005) In our search for potential new anticancer drugs, we designed and synthesized a series of tricyclic compounds containing the antimitotic 2-phenylazaflavone chromophore fused to a pyrrole ring in a pyr... |
|
|
Cytokinin activity of azaindene, azanaphthalene, naphthalene, and indole derivatives. Torigoe Y, et al.
Phytochemistry 11(5) , 1623-1630, (1972)
|
|
|
Synthestic approaches to the teleocidin-related tumour promoters: a total synthesis of (±)-indolactam V. de Laszlo SE, et al.
J. Chem. Soc. Chem. Commun. 4 , 344-346., (1986)
|
| 1H-Indol-4-ylamine |
| 4-Aminoindole |
| 4-Indolamine |
| indole-4-ylamine |
| (Indol-4-yl)amine |
| 4-amino-1h-indole |
| MFCD01076559 |
| 6-amino-1H-indole |
| 1h-indol-4-amin |
| 1H-indole-4-amine |
| 1H-Indol-4-amine |
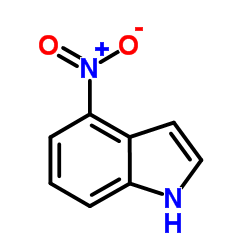 CAS#:4769-97-5
CAS#:4769-97-5![3-nitro-trans-2-[β-(dimethylamino)vinyl]-nitrobenzene Structure](https://image.chemsrc.com/caspic/138/78283-21-3.png) CAS#:78283-21-3
CAS#:78283-21-3 CAS#:7439-89-6
CAS#:7439-89-6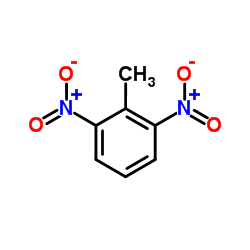 CAS#:606-20-2
CAS#:606-20-2 CAS#:78283-23-5
CAS#:78283-23-5 CAS#:52537-01-6
CAS#:52537-01-6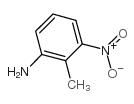 CAS#:603-83-8
CAS#:603-83-8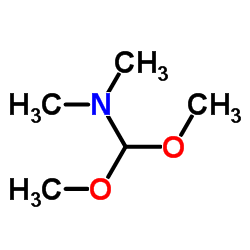 CAS#:4637-24-5
CAS#:4637-24-5 CAS#:2942-36-1
CAS#:2942-36-1 CAS#:135531-91-8
CAS#:135531-91-8 CAS#:135531-89-4
CAS#:135531-89-4 CAS#:84807-09-0
CAS#:84807-09-0 CAS#:885270-30-4
CAS#:885270-30-4 CAS#:2380-94-1
CAS#:2380-94-1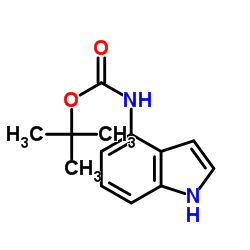 CAS#:819850-13-0
CAS#:819850-13-0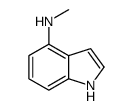 CAS#:85696-93-1
CAS#:85696-93-1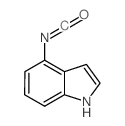 CAS#:581812-74-0
CAS#:581812-74-0
