3',4',7-trihydroxy isoflavone
Modify Date: 2024-01-03 00:15:34
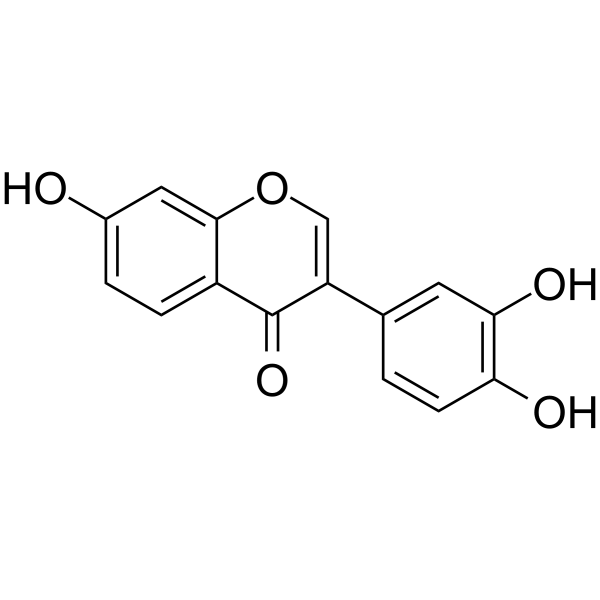
3',4',7-trihydroxy isoflavone structure
|
Common Name | 3',4',7-trihydroxy isoflavone | ||
|---|---|---|---|---|
| CAS Number | 485-63-2 | Molecular Weight | 270.24 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 572.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C15H10O5 | Melting Point | 280-282°C | |
| MSDS | N/A | Flash Point | 224.0±23.6 °C | |
Use of 3',4',7-trihydroxy isoflavone7,3',4'-Trihydroxyisoflavone, a major metabolite of Daidzein, is an ATP-competitive inhibitor of Cot (Tpl2/MAP3K8) and MKK4. 7,3',4'-Trihydroxyisoflavone has anticancer, anti-angiogenic, chemoprotective, and free radical scavenging activities[1][2]. |
| Name | 3',4',7-trihydroxyisoflavone |
|---|---|
| Synonym | More Synonyms |
| Description | 7,3',4'-Trihydroxyisoflavone, a major metabolite of Daidzein, is an ATP-competitive inhibitor of Cot (Tpl2/MAP3K8) and MKK4. 7,3',4'-Trihydroxyisoflavone has anticancer, anti-angiogenic, chemoprotective, and free radical scavenging activities[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 7,3',4'-Trihydroxyisoflavone triggers cell cycle arrest at the G1 phase and displays an anti-proliferative effect against EGF receptor-positive skin cancer[1]. 7,3',4'-Trihydroxyisoflavone also significantly inhibits UVB-induced COX-2 expression by suppressing the NF-B transcription activity in mouse skin epidermal JB6 P+ cells[1]. |
| In Vivo | In a mouse skin tumorigenesis model, 7,3',4'-Trihydroxyisoflavone strongly suppresses the incidence, multiplicity, and volume of UVB-induced mouse skin tumors. Consistent with the tumor data, 7,3',4'-Trihydroxyisoflavone clearly attenuates UVB-induced COX-2 expression in hairless mouse skin[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 572.8±50.0 °C at 760 mmHg |
| Melting Point | 280-282°C |
| Molecular Formula | C15H10O5 |
| Molecular Weight | 270.24 |
| Flash Point | 224.0±23.6 °C |
| Exact Mass | 270.052826 |
| PSA | 90.90000 |
| LogP | 2.58 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.732 |
| Storage condition | -20°C Freezer |
| Stability | Store in Freezer |
| Risk Phrases | R36/37/38 |
|---|---|
| Safety Phrases | S22-S24/25 |
| WGK Germany | 3 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| 7,3',4'-THIF |
| 3-(3,4-Dihydroxyphenyl)-7-hydroxy-4H-chromen-4-one |
| 3',4',7-Trihydroxyisoflavone |
| 3`,4`,7-Trihydroxyisoflavone |
| 4H-1-Benzopyran-4-one, 3-(3,4-dihydroxyphenyl)-7-hydroxy- |
| 3l5r |
| MFCD00143002 |
| 3-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-on |
| 3',4',7-trihydroxy isoflavone |
| 3'-Hydroxydaidzein |
| 3-(3,4-dihydroxyphenyl)-7-hydroxychromen-4-one |
| 3-(3,4-dihydroxy-phenyl)-7-hydroxy-chromen-4-one |
| 7,3',4'-Trihydroxyisoflavone |
| 7-Hydroxy-3-(3,4-dihydroxyphenyl)-4H-chromen-4-one |
| 4H-1-Benzopyran-4-one,3-(3,4-dihydroxyphenyl)-7-hydroxy |
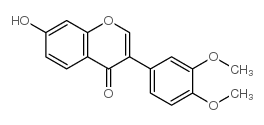 CAS#:24160-14-3
CAS#:24160-14-3 CAS#:887354-66-7
CAS#:887354-66-7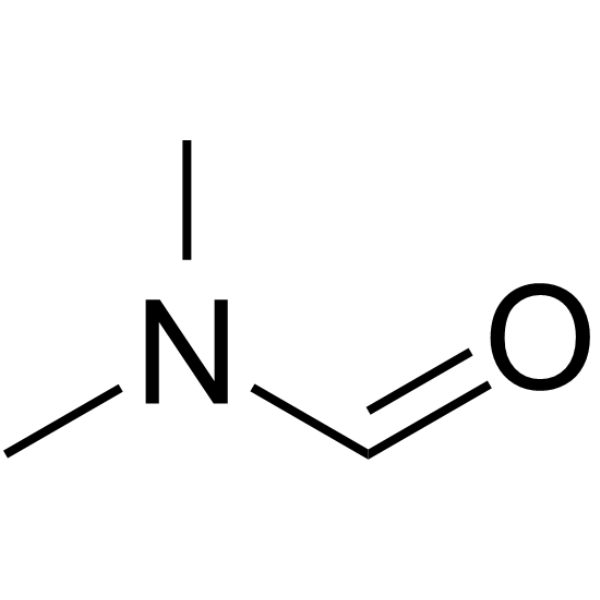 CAS#:68-12-2
CAS#:68-12-2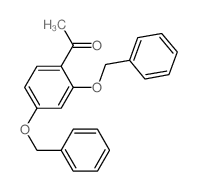 CAS#:22877-01-6
CAS#:22877-01-6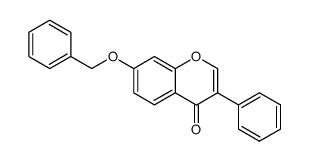 CAS#:4253-04-7
CAS#:4253-04-7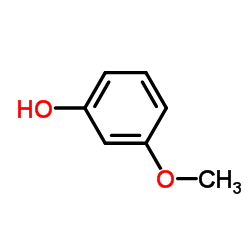 CAS#:150-19-6
CAS#:150-19-6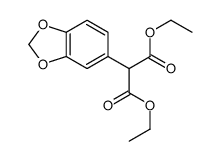 CAS#:77069-04-6
CAS#:77069-04-6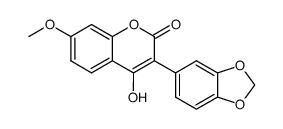 CAS#:77069-05-7
CAS#:77069-05-7 CAS#:1621-61-0
CAS#:1621-61-0