16,16-Dimethyl prostaglandin E2
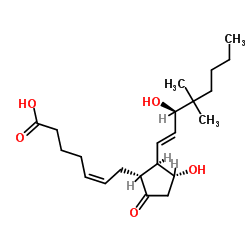
16,16-Dimethyl prostaglandin E2 structure
|
Common Name | 16,16-Dimethyl prostaglandin E2 | ||
|---|---|---|---|---|
| CAS Number | 39746-25-3 | Molecular Weight | 380.518 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 541.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C22H36O5 | Melting Point | -85.6ºC | |
| MSDS | Chinese USA | Flash Point | 295.2±26.6 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of 16,16-Dimethyl prostaglandin E216,16-Dimethyl prostaglandin E2 (16,16-dimethyl PGE2) is an orally active vertebrate Hematopoietic stem cells (HSCs) homeostasis critical regulator. 16,16-Dimethyl prostaglandin E2 can act through EP2/EP4 and has an interaction with the Wnt pathway[1][2]. |
| Name | 16,16-Dimethylprostaglandin E2 |
|---|---|
| Synonym | More Synonyms |
| Description | 16,16-Dimethyl prostaglandin E2 (16,16-dimethyl PGE2) is an orally active vertebrate Hematopoietic stem cells (HSCs) homeostasis critical regulator. 16,16-Dimethyl prostaglandin E2 can act through EP2/EP4 and has an interaction with the Wnt pathway[1][2]. |
|---|---|
| Related Catalog | |
| Target |
EP2 EP4 |
| In Vivo | 16,16-Dimethyl prostaglandin E2 (16,16-dimethyl PGE2; 10 µg/kg; i.p.; every 12 hours; for 8 days) rescues the exacerbated DSS colitis phenotype of Tpl2IMF-KO mice and restores epithelial proliferation and structure[2]. Animal Model: Tpl2IMF-KO mice[2] Dosage: 10 µg/kg Administration: IP; every 12 hours; for 8 days Result: Rescued the exacerbated DSS colitis phenotype and restores epithelial proliferation and structure. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 541.3±50.0 °C at 760 mmHg |
| Melting Point | -85.6ºC |
| Molecular Formula | C22H36O5 |
| Molecular Weight | 380.518 |
| Flash Point | 295.2±26.6 °C |
| Exact Mass | 380.256287 |
| PSA | 94.83000 |
| LogP | 2.57 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.551 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H319-H336 |
| Supplemental HS | Repeated exposure may cause skin dryness or cracking. |
| Precautionary Statements | P210-P280-P304 + P340 + P312-P305 + P351 + P338-P337 + P313-P403 + P235 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | F,Xn,Xi |
| Risk Phrases | 11-20/21/22-36/37/38-67-66-36 |
| Safety Phrases | 16-26-36 |
| RIDADR | UN 1231 3/PG 2 |
|
~% 
16,16-Dimethyl ... CAS#:39746-25-3 |
| Literature: Rodriguez, Ana; Nomen, Miguel; Spur, Bernd W.; Godfroid, Jean-Jacques Archiv der Pharmazie, 1998 , vol. 331, # 9 p. 279 - 282 |
|
~% 
16,16-Dimethyl ... CAS#:39746-25-3 |
| Literature: Rodriguez, Ana; Nomen, Miguel; Spur, Bernd W.; Godfroid, Jean-Jacques Archiv der Pharmazie, 1998 , vol. 331, # 9 p. 279 - 282 |
|
~% 
16,16-Dimethyl ... CAS#:39746-25-3 |
| Literature: Rodriguez, Ana; Nomen, Miguel; Spur, Bernd W.; Godfroid, Jean-Jacques Archiv der Pharmazie, 1998 , vol. 331, # 9 p. 279 - 282 |
|
~% 
16,16-Dimethyl ... CAS#:39746-25-3 |
| Literature: Rodriguez, Ana; Nomen, Miguel; Spur, Bernd W.; Godfroid, Jean-Jacques Archiv der Pharmazie, 1998 , vol. 331, # 9 p. 279 - 282 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
|
Lysophosphatidic acid enhances survival of human CD34(+) cells in ischemic conditions.
Sci. Rep. 5 , 16406, (2015) Several clinical trials are exploring therapeutic effect of human CD34(+) cells in ischemic diseases, including myocardial infarction. Unfortunately, most of the cells die few days after delivery. Her... |
|
|
Prostaglandin E2--mediated relaxation of the ductus arteriosus: effects of gestational age on g protein-coupled receptor expression, signaling, and vasomotor control.
Circulation 110(16) , 2326-32, (2004) In the preterm newborn, a patent ductus arteriosus is in large part a result of the increased sensitivity of the immature ductus to prostaglandin E2 (PGE2). PGE2 acts through 3 G protein-coupled recep... |
|
|
Prostanoids regulate angiogenesis acting primarily on IP and EP4 receptors.
Microvasc. Res. 101 , 127-34, (2015) Angiogenesis is regulated by numerous activators and inhibitors, including prostanoids. Although many studies have identified their roles in inflammation, regulatory functions of prostanoids in angiog... |
| (5Z,11α,13E,15R)-11,15-Dihydroxy-16,16-dimethyl-9-oxoprosta-5,13-dien-1-oic acid |
| Prosta-5,13-dien-1-oic acid, 11,15-dihydroxy-16,16-dimethyl-9-oxo-, (5Z,11α,13E,15R)- |
| 16,16-Dimethyl Prostaglandin E2,(5Z,11α,13E,15R)-11,15-Dihydroxy-16,16-dimethyl-9-oxo-prosta-5,13-dien-1oicacid |
![methyl (Z)-7-[(1R,2R,3R)-3-hydroxy-2-[(E)-3-hydroxy-4,4-dimethyloct-1-enyl]-5-oxocyclopentyl]hept-5-enoate structure](https://image.chemsrc.com/caspic/321/42782-96-7.png)

 CAS#:41691-92-3
CAS#:41691-92-3