3,5-Di-tert-butyl-2-hydroxybenzaldehyde

3,5-Di-tert-butyl-2-hydroxybenzaldehyde structure
|
Common Name | 3,5-Di-tert-butyl-2-hydroxybenzaldehyde | ||
|---|---|---|---|---|
| CAS Number | 37942-07-7 | Molecular Weight | 234.334 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 277.6±35.0 °C at 760 mmHg | |
| Molecular Formula | C15H22O2 | Melting Point | 59-61 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 116.1±18.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 3,5-Bis(1,1-dimethylethyl)-2-hydroxy-benzaldehyde |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 277.6±35.0 °C at 760 mmHg |
| Melting Point | 59-61 °C(lit.) |
| Molecular Formula | C15H22O2 |
| Molecular Weight | 234.334 |
| Flash Point | 116.1±18.5 °C |
| Exact Mass | 234.161987 |
| PSA | 37.30000 |
| LogP | 4.98 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.528 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2912499000 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2912499000 |
|---|---|
| Summary | 2912499000. other aldehyde-ethers, aldehyde-phenols and aldehydes with other oxygen function. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Synthesis, characterization and electrochemical study of synthesis of a new Schiff base (H₂cddi(t)butsalen) ligand and their two asymmetric Schiff base complexes of Ni(II) and Cu(II) with NN'OS coordination spheres.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 97 , 1033-40, (2012) A novel Schiff base (H(2)cddi(t)butsalen) ligand was prepared via condensation of Methyl-2-{N-(2'-aminoethane)}-amino-1-cyclopentenedithiocarboxylate(Hcden) and 3,5-di-tert-butyl-2-hydroxybenzaldehyde... |
|
|
Novel Organotin(IV) Schiff Base Complexes with Histidine Derivatives: Synthesis, Characterization, and Biological Activity.
Bioinorg. Chem. Appl. 2013 , 502713, (2013) Five novel tin Schiff base complexes with histidine analogues (derived from the condensation reaction between L-histidine and 3,5-di-tert-butyl-2-hydroxybenzaldehyde) have been synthesized and charact... |
|
|
Tetrahedron Asymmetry 18 , 1124, (2007)
|
| MFCD00191998 |
| 3,5-di-tert-butyl-2-hydroxybenzaldehyde |
| 3,5-di-tert-Butyl Salicylaldehyde |
| 3,5-Di-tert-butyl-2-hydroxybenzolcarbaldehyd |
| 3,5-Di-tert-butylsalicylaldehyde |
| 3,5-Bis(1,1-Dimethylethyl)-2-Hydroxy-Benzaldehyde |
| Benzaldehyde, 3,5-bis(1,1-dimethylethyl)-2-hydroxy- |
| 3,5-ditert-butyl-2-hydroxybenzaldehyde |
| 2-Hydroxy-3,5-bis(2-methyl-2-propanyl)benzaldehyde |
 CAS#:50-00-0
CAS#:50-00-0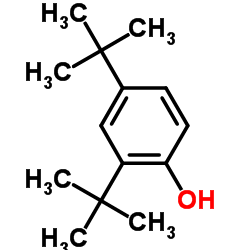 CAS#:96-76-4
CAS#:96-76-4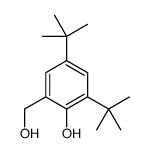 CAS#:16373-02-7
CAS#:16373-02-7 CAS#:32857-07-1
CAS#:32857-07-1 CAS#:108-95-2
CAS#:108-95-2 CAS#:67-56-1
CAS#:67-56-1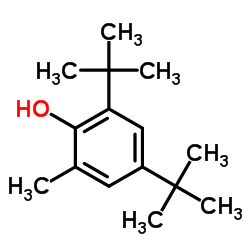 CAS#:616-55-7
CAS#:616-55-7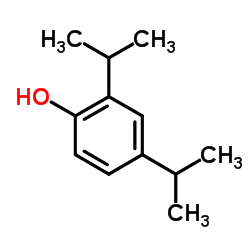 CAS#:2934-05-6
CAS#:2934-05-6 CAS#:100-97-0
CAS#:100-97-0 CAS#:143569-55-5
CAS#:143569-55-5![2,4-ditert-butyl-6-[(pyridin-2-ylmethylamino)methyl]phenol structure](https://image.chemsrc.com/caspic/193/213775-52-1.png) CAS#:213775-52-1
CAS#:213775-52-1![2,4-ditert-butyl-6-[(4-methylanilino)methylidene]cyclohexa-2,4-dien-1-one structure](https://image.chemsrc.com/caspic/495/154289-86-8.png) CAS#:154289-86-8
CAS#:154289-86-8![2,4-ditert-butyl-6-[(2-methoxyanilino)methylidene]cyclohexa-2,4-dien-1-one structure](https://image.chemsrc.com/caspic/164/154289-77-7.png) CAS#:154289-77-7
CAS#:154289-77-7 CAS#:135546-15-5
CAS#:135546-15-5![2,4-ditert-butyl-6-[(2-hydroxyanilino)methylidene]cyclohexa-2,4-dien-1-one structure](https://image.chemsrc.com/caspic/005/319482-73-0.png) CAS#:319482-73-0
CAS#:319482-73-0![2,4-ditert-butyl-6-[(propan-2-ylamino)methylidene]cyclohexa-2,4-dien-1-one structure](https://image.chemsrc.com/caspic/392/345300-65-4.png) CAS#:345300-65-4
CAS#:345300-65-4 CAS#:135616-40-9
CAS#:135616-40-9 CAS#:135616-36-3
CAS#:135616-36-3 CAS#:174022-08-3
CAS#:174022-08-3
