Tyrphostin 8
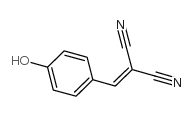
Tyrphostin 8 structure
|
Common Name | Tyrphostin 8 | ||
|---|---|---|---|---|
| CAS Number | 3785-90-8 | Molecular Weight | 170.17 | |
| Density | 1.29 g/cm3 | Boiling Point | 354.3ºC at 760 mmHg | |
| Molecular Formula | C10H6N2O | Melting Point | 186-189 °C | |
| MSDS | Chinese USA | Flash Point | 168.1ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Tyrphostin 8Tyrphostin 8 is a tyrosine kinase, with an IC50 of 560 μM for EGFR kinase. Tyrphostin 8 is also a GTPase inhibitor. Tyrphostin 8 can inhibit the protein serine/threonine phosphatase calcineurin (IC50=21 μM)[1][2][3]. |
| Name | 4-hydroxybenzylidenemalononitrile |
|---|---|
| Synonym | More Synonyms |
| Description | Tyrphostin 8 is a tyrosine kinase, with an IC50 of 560 μM for EGFR kinase. Tyrphostin 8 is also a GTPase inhibitor. Tyrphostin 8 can inhibit the protein serine/threonine phosphatase calcineurin (IC50=21 μM)[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
EGFR:560 μM (IC50) calcineuin phosphatase:21 μM (IC50) |
| In Vitro | Tyrphostin 8 (10-100 μM; pretreated for 20 min) blocks the Carbachol-initiated PKCδ tyrosine phosphorylation and ERK1/2 activation in parotid acinar cells[1]. Tyrphostin 8 (10-100 μM) produces a rapid and large increase in the basal O2 consumption of parotid acinar[1]. Tyrphostin 8 (10-100 μM) reduces the parotid ATP content by ∼90% at the concentration of 100 μM[1]. Tyrphostin 8 increases apical-to-basolateral transport of insulin-transferrin conjugate by enhancing transferrin receptor-mediated transcytosis in filter-grown Caco-2 cell monolayer[2]. Western Blot Analysis[1] Cell Line: Parotid acinar cells Concentration: 10, 100 μM Incubation Time: Pretreated for 20 min Result: Reduced the increase in tyrosine phosphorylation of PKCδ initiated by carbachol. Reduced the activation of ERK1/2 by carbachol. |
| In Vivo | Tyrphostin 8 improves the glucose-lowering effect of Insulin-transferrin in Streptozotocin-induced diabetic rats[2]. |
| References |
| Density | 1.29 g/cm3 |
|---|---|
| Boiling Point | 354.3ºC at 760 mmHg |
| Melting Point | 186-189 °C |
| Molecular Formula | C10H6N2O |
| Molecular Weight | 170.17 |
| Flash Point | 168.1ºC |
| Exact Mass | 170.04800 |
| PSA | 67.81000 |
| LogP | 1.82276 |
| Index of Refraction | 1.653 |
| Storage condition | 2-8°C |
| Water Solubility | ethanol: 20 mg/mL, clear |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H319 |
| Precautionary Statements | P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | 25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | OO4200000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2926909090 |
|
~98% 
Tyrphostin 8 CAS#:3785-90-8 |
| Literature: Gopalakrishna Panicker, Rajesh Krishnan; Krishnapillai, Sreekumar Tetrahedron Letters, 2014 , vol. 55, # 15 p. 2352 - 2354 |
|
~59% 
Tyrphostin 8 CAS#:3785-90-8 |
| Literature: Sidhu, Anjali; Sharma; Rai, Mangat Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2010 , vol. 49, # 2 p. 247 - 250 |
|
~% 
Tyrphostin 8 CAS#:3785-90-8 |
| Literature: Ertel,W.; Friedrich,K. Chemische Berichte, 1977 , vol. 110, p. 86 - 95 |
|
~% 
Tyrphostin 8 CAS#:3785-90-8 |
| Literature: Ertel,W.; Friedrich,K. Chemische Berichte, 1977 , vol. 110, p. 86 - 95 |
|
~% 
Tyrphostin 8 CAS#:3785-90-8
Detail
|
| Literature: Maggi, Raimondo; Ballini, Roberto; Sartori, Giovanni; Sartorio, Raffaella Tetrahedron Letters, 2004 , vol. 45, # 11 p. 2297 - 2299 |
| Precursor 6 | |
|---|---|
| DownStream 5 | |
| HS Code | 2926909090 |
|---|---|
| Summary | HS:2926909090 other nitrile-function compounds VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Effect of combined changes in delayed extraction time and potential gradient on the mass resolution and ion discrimination in the analysis of polydisperse polymers and polymer blends by delayed extraction matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Rapid Commun. Mass Spectrom. 13(24) , 2511-7, (1999) Data reported here show that, in the delayed extraction matrix-assisted laser desorption/ionization time-of-flight (DE-MALDI-TOF) mass spectrometric analysis of synthetic polydisperse polymers, differ... |
|
|
[Prevalence of Aeromonas spp. pediatric gastroenteritis].
CMAJ 138(8) , 714-7, (1988) A selective medium and biochemical tests were used to search for Aeromonas spp. in the stools of 536 children, more than 90% of whom had "gastroenteritis", seen at Sainte-Justine Hospital, Montreal, i... |
|
|
Characterization and modulation of the transferrin receptor on brain capillary endothelial cells.
Pharm. Res. 21(5) , 761-9, (2004) The expression level of the transferrin receptor (TfR) on brain capillary endothelial cells (BCECs) and the endocytosis of 125I-transferrin (125I-Tf) by this receptor was investigated. Furthermore, th... |
| 4-(HYDROXYBENZYLIDENE)-MALONONITRILE |
| 4-Hydroxybenzylidenemalononitrile |
| (4-Hydroxybenzylidene)malonitrile |
| EINECS 223-253-0 |
| 2-[(4-hydroxyphenyl)methylidene]propanedinitrile |
| MFCD00020189 |

 CAS#:5553-97-9
CAS#:5553-97-9![[4-(2,2-dicyanoethenyl)phenyl] acetate structure](https://image.chemsrc.com/caspic/128/19310-87-3.png) CAS#:19310-87-3
CAS#:19310-87-3 CAS#:122520-81-4
CAS#:122520-81-4 CAS#:6504-13-8
CAS#:6504-13-8![[4-(2,2-dicyanoethenyl)phenyl] 4-heptanoyloxybenzoate structure](https://image.chemsrc.com/caspic/002/78016-54-3.png) CAS#:78016-54-3
CAS#:78016-54-3