SU 9516
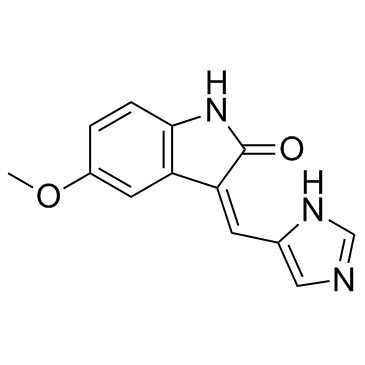
SU 9516 structure
|
Common Name | SU 9516 | ||
|---|---|---|---|---|
| CAS Number | 377090-84-1 | Molecular Weight | 241.245 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 601.5±55.0 °C at 760 mmHg | |
| Molecular Formula | C13H11N3O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 317.6±31.5 °C | |
Use of SU 9516SU9516 is a potent CDK2 inhibitor, with an IC50 of 22 nM, and also shows inhibitory effects on CDK1 and CDK4, with IC50s of 40, 200 nM, respectively. |
| Name | 3-(1H-imidazol-5-ylmethylidene)-5-methoxy-1H-indol-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | SU9516 is a potent CDK2 inhibitor, with an IC50 of 22 nM, and also shows inhibitory effects on CDK1 and CDK4, with IC50s of 40, 200 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
CDK2:22 nM (IC50) CDK1:40 nM (IC50) CDK4:200 nM (IC50) PDGFr:18000 nM (IC50) |
| In Vitro | SU9516 shows slight activities against PKC, p38, PDGFr and EGFR, with IC50 of >10, >10, 18, and >100 μM. SU9516 (5 μM) decreases cdk2-specific Phosphorylation of pRB and inhibits cell cycle progression in RKO cells. SU9516 (5 μM) also induces apoptosis in RKO and SW480 Cells[1]. SU9516 (5 μM) results in enhanced pRb/E2F complex formation in HT-29 cells. SU9516 enhances presence of E2F species in multiprotein complexes[2]. SU9516 (5 μM) rapidly induces cytochrome crelease, Bax mitochondrial translocation, and apoptosis in association with pronounced down-regulation of the antiapoptotic protein Mcl-1. SU9516 causes down-regulation of Mcl-1 mRNA levels in human leukemia cells. Furthermore, SU9516 treatment results in a marked increase in reactive oxygen species production[3]. |
| Kinase Assay | Kinase assays are performed in 96-well polypropylene plates. Each reaction contains 2 μg of histone H1 at a final concentration of 10 μM [-33P]ATP (0.2 μCi/well), 10 mM MgCl2,1mM DTT, 0.01% Triton X-100, and 10% glycerol in a 40 μL volume. The reaction is initiated with the addition of 20 μL enzyme (6 ng cdk2/well resulting in a final concentration of 1.6 nM), which is previously diluted 1:50-1:200 in the same buffer, and allowed to proceed for 1 h at room temperature. Reaction is stopped by the addition of 0.01 mL 10% phosphoric acid, and 25 μL of reaction mixture is transferred to P30 phosphocellulose filter mat paper. The filter mat is washed three times with 1.0% phosphoric acid, air dried, and then counted for radioactivity in a liquid scintillation counter. |
| Cell Assay | RKO cells and SW480 cells are seeded in replicates (n = 6) in 96-well plates at 1×104 cells/well and allowed to attach overnight. SU9516 is added in concentrations from 0.05 μM to 50.00 μM for 24 h, the cells are then washed twice with PBS, and cells are replenished with complete media. The cells are fixed at 0, 4, and 7 days post-drug removal and assayed for protein levels using a modified SRB cytotoxicity assay. The cells are fixed in 10% trichloroacetic acid for 1 h, washed in distilled H2O, and stained in 0.4% SRB/acetic acid for 30 min. The cells are then washed in 0.1% acetic acid, solubilized in 10 mM Tris (pH 9), and analyzed on a Bio-Rad 360 microplate reader at 595 nm. All experiments are repeated at least three times. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 601.5±55.0 °C at 760 mmHg |
| Molecular Formula | C13H11N3O2 |
| Molecular Weight | 241.245 |
| Flash Point | 317.6±31.5 °C |
| Exact Mass | 241.085129 |
| PSA | 67.01000 |
| LogP | 1.02 |
| Appearance of Characters | crystalline | brown |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.696 |
| InChIKey | QNUKRWAIZMBVCU-WCIBSUBMSA-N |
| SMILES | COc1ccc2c(c1)C(=Cc1cnc[nH]1)C(=O)N2 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: ~17 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
The FGF-1-specific single-chain antibody scFv1C9 effectively inhibits breast cancer tumour growth and metastasis.
J. Cell. Mol. Med. 18(10) , 2061-70, (2014) Immunotherapy mediated by recombinant antibodies is an effective therapeutic strategy for a variety of cancers. In a previous study, we demonstrated that the fibroblast growth factor 1 (FGF-1)-specifi... |
|
|
The three-substituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516) kills human leukemia cells via down-regulation of Mcl-1 through a transcriptional mechanism.
Mol. Pharmacol. 70(2) , 645-55, (2006) Mechanisms of lethality of the three-substituted indolinone and putatively selective cyclin-dependent kinase (CDK)2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-on... |
|
|
You never know: Cdk inhibitors as anti-cancer drugs.
Cell Cycle 7(24) , 3789-90, (2008) Ever since it emerged that cyclin-dependent protein kinases catalyzed cell cycle transitions and with cancer seen as "a disease of the cell cycle," people have pursued the aim of testing kinase inhibi... |
| Kinome_2853 |
| 2H-Indol-2-one, 1,3-dihydro-3-(1H-imidazol-5-ylmethylene)-5-methoxy-, (3Z)- |
| (3Z)-3-(1H-Imidazol-4-ylmethylene)-5-methoxy-1,3-dihydro-2H-indol-2-one |
| SU9516 |