1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-6-iodohexane
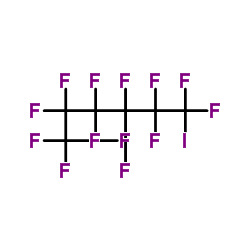
1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-6-iodohexane structure
|
Common Name | 1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-6-iodohexane | ||
|---|---|---|---|---|
| CAS Number | 355-43-1 | Molecular Weight | 445.948 | |
| Density | 2.0±0.1 g/cm3 | Boiling Point | 117.1±8.0 °C at 760 mmHg | |
| Molecular Formula | C6F13I | Melting Point | -45 °C | |
| MSDS | Chinese USA | Flash Point | 45.0±5.6 °C | |
| Name | Perfluoro-1-iodohexane |
|---|---|
| Synonym | More Synonyms |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 117.1±8.0 °C at 760 mmHg |
| Melting Point | -45 °C |
| Molecular Formula | C6F13I |
| Molecular Weight | 445.948 |
| Flash Point | 45.0±5.6 °C |
| Exact Mass | 445.883698 |
| LogP | 7.22 |
| Vapour Pressure | 21.1±0.2 mmHg at 25°C |
| Index of Refraction | 1.333 |
| Stability | Stable. |
| Water Solubility | insoluble |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S23-S24/25-S37/39-S26 |
| RIDADR | 2810 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29034700 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2903799090 |
|---|---|
| Summary | 2903799090 halogenated derivatives of acyclic hydrocarbons containing two or more different halogens。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Transition metal complexes bearing NHC ligands substituted with secondary polyfluoroalkyl groups.
Dalton Trans. 44 , 19663-73, (2015) Using three different approaches, racemic 1-(perfluoroalkyl)ethylamines were synthesized from perfluoroalkyl iodides or perfluoroalkanoic acids, and further transformed to the corresponding N,N'-disub... |
|
|
The in vitro estrogenic activities of polyfluorinated iodine alkanes.
Environ. Health Perspect. 120(1) , 119-25, (2012) Polyfluorinated iodine alkanes (PFIs) are important intermediates in the synthesis of organic fluoride products. Recently, PFIs have been detected in fluoropolymers as residual raw materials, as well ... |
|
|
Non-covalent interactions between iodo-perfluorocarbons and hydrogen bond acceptors.
Chem. Commun. (Camb.) (15) , 2005-7, (2009) Quantitative studies of the 1 : 1 complexes formed between perfluorohexyl iodide and a variety of hydrogen-bond acceptors have been used to probe the relationship between halogen bonding, hydrogen bon... |
| 1,1,2,2-Tetrahydroperfluorohexyl iodide |
| 1-Iodotridecafluorohexane |
| Tridecafluoro-1-iodohexane |
| Hexane, 1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluoro-6-iodo- |
| Perfluorohexyl iodide |
| 1-iodo-tridecafluoro-hexane |
| 1,1,2,2-tetrahydroperfluorohexyliodide |
| Perfluoro-1-iodohexane |
| Perfluorohexyliodide |
| 1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluoro-1-iodohexane |
| 1,1,1,2,2,3,3,4,4,5,5,6,6-Tridecafluoro-6-iodohexane |
| 1-Iodoperfluorohexane |
| MFCD00001063 |
| Perfluoro-n-hexyl iodide |
| EINECS 206-586-6 |
| TRIDECAFLUOROHEXYL IODIDE |
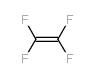 CAS#:116-14-3
CAS#:116-14-3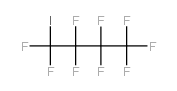 CAS#:423-39-2
CAS#:423-39-2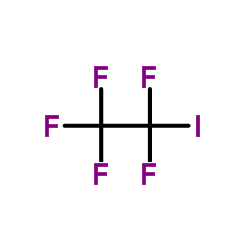 CAS#:354-64-3
CAS#:354-64-3 CAS#:4520-08-5
CAS#:4520-08-5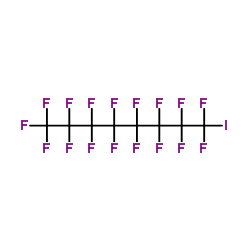 CAS#:507-63-1
CAS#:507-63-1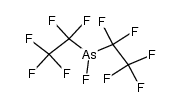 CAS#:32395-49-6
CAS#:32395-49-6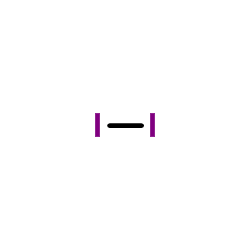 CAS#:7553-56-2
CAS#:7553-56-2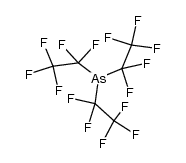 CAS#:359-69-3
CAS#:359-69-3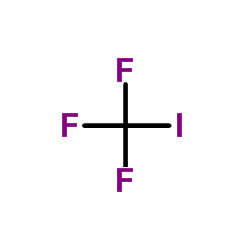 CAS#:2314-97-8
CAS#:2314-97-8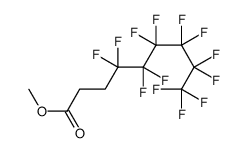 CAS#:110260-75-8
CAS#:110260-75-8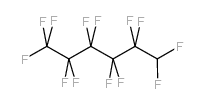 CAS#:355-37-3
CAS#:355-37-3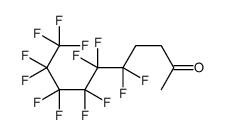 CAS#:110388-12-0
CAS#:110388-12-0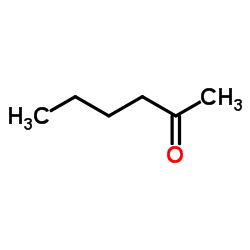 CAS#:591-78-6
CAS#:591-78-6 CAS#:104698-09-1
CAS#:104698-09-1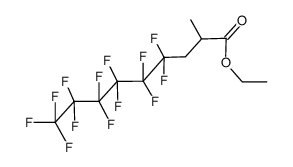 CAS#:140834-68-0
CAS#:140834-68-0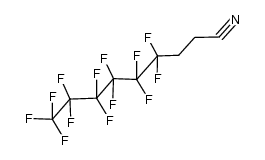 CAS#:26649-25-2
CAS#:26649-25-2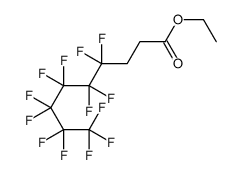 CAS#:60023-26-9
CAS#:60023-26-9 CAS#:140834-72-6
CAS#:140834-72-6 CAS#:2923-26-4
CAS#:2923-26-4
