Homobutein
Modify Date: 2025-08-25 20:38:41
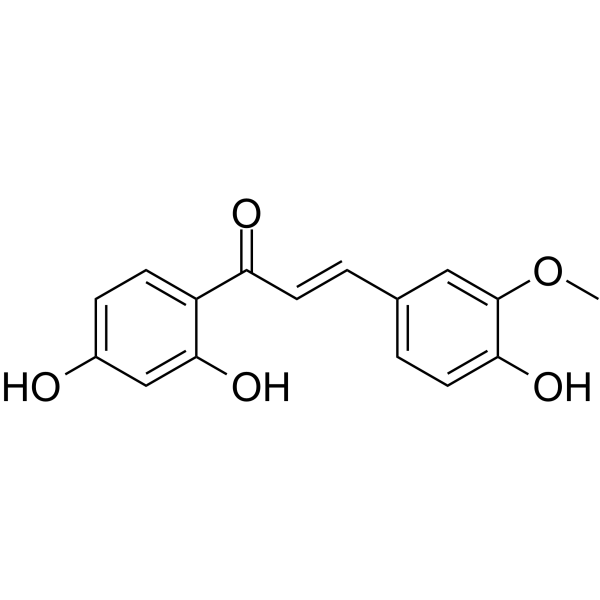
Homobutein structure
|
Common Name | Homobutein | ||
|---|---|---|---|---|
| CAS Number | 34000-39-0 | Molecular Weight | 286.27900 | |
| Density | 1.369 g/cm3 | Boiling Point | 523.8ºC at 760 mmHg | |
| Molecular Formula | C16H14O5 | Melting Point | 207-212ºC | |
| MSDS | N/A | Flash Point | 197.6ºC | |
Use of HomobuteinHomobutein a natural chalcones (can be found in many medicinal plants, fruits, vegetables, spices and nuts), is a potent HDACs/NF-κB dual inhibitor with IC50s of 190 and 38 μM, respectively. Homobutein also a chelator of iron (II and III) cations, shows various activities, including anticancer, anti-inflammatory, antiparasite and antioxidation[1][2][3][4]. |
| Name | (E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one |
|---|---|
| Synonym | More Synonyms |
| Description | Homobutein a natural chalcones (can be found in many medicinal plants, fruits, vegetables, spices and nuts), is a potent HDACs/NF-κB dual inhibitor with IC50s of 190 and 38 μM, respectively. Homobutein also a chelator of iron (II and III) cations, shows various activities, including anticancer, anti-inflammatory, antiparasite and antioxidation[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Homobutein (compound 15) (20, 24, 28, 32, 40 μM; 2 h) inhibits the viability of K562 cells[1]. Homobutein (2 h) inhibits TNFα-induced NF-κB activity in K562 cells[1]. Homobutein (1 μg/mL; 72 h) inhibits the growth of Toxoplasma gondii by 19.48%[2]. Homobutein (24 h) againsts W2 and D6 strains of P.falciparum with IC50s of 15.0 and 16.1 µM, respectively[3]. Cell Viability Assay[1] Cell Line: K562 cells Concentration: 20, 24, 28, 32, 40 μM Incubation Time: 2 h Result: Showed inhibition of viability in K562 cells. Cell Viability Assay[2] Cell Line: Toxoplasma gondii RH-2F strain Concentration: 1 μg/mL Incubation Time: 72 h Result: Surpressed 19.48% of the Toxoplasma gondii. |
| References |
| Density | 1.369 g/cm3 |
|---|---|
| Boiling Point | 523.8ºC at 760 mmHg |
| Melting Point | 207-212ºC |
| Molecular Formula | C16H14O5 |
| Molecular Weight | 286.27900 |
| Flash Point | 197.6ºC |
| Exact Mass | 286.08400 |
| PSA | 86.99000 |
| LogP | 2.70810 |
| Index of Refraction | 1.684 |
| HS Code | 2914509090 |
|---|
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
| 2',4,4'-trihydroxy-3-methoxychalcone |
| 2-Propen-1-one,1-(2,4-dihydroxyphenyl)-3-(4-hydroxy-3-methoxyphenyl) |
| 3-O-Methylbutein |
| 1-(2,4-Dihydroxyphenyl)-3-(4-hydroxy-3-methoxyphenyl)-2-propen-1-one |
| (2E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one |
| EINECS 251-782-7 |
| 2',4,4'-trihydroxy-3-dimethoxychalcone |
| 4,2',4'-trihydroxy-3-methoxy-trans-chalcone |
| Acrylophenone,2',4'-dihydroxy-3-(p-hydroxy-m-methoxyphenyl) |
| 4,2',4'-Trihydroxy-3-methoxy-trans-chalkon |
| Homobutein |
 CAS#:89-84-9
CAS#:89-84-9 CAS#:121-33-5
CAS#:121-33-5 CAS#:67756-04-1
CAS#:67756-04-1 CAS#:5460-32-2
CAS#:5460-32-2![1-[2-hydroxy-4-(methoxymethoxy)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/486/65490-08-6.png) CAS#:65490-08-6
CAS#:65490-08-6 CAS#:5533-00-6
CAS#:5533-00-6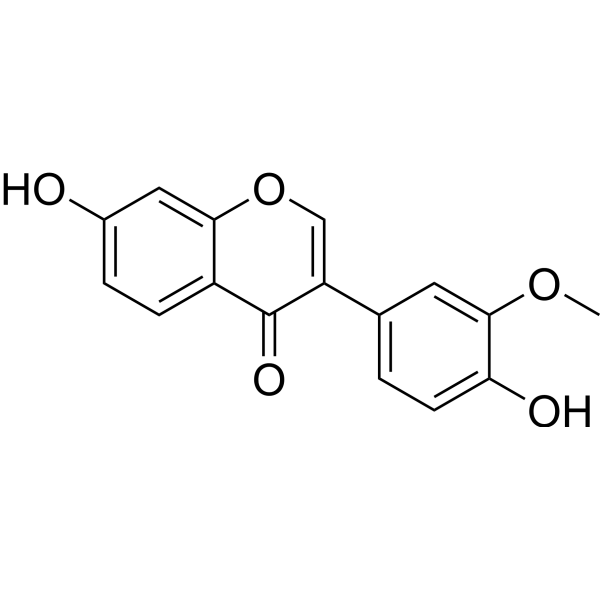 CAS#:21913-98-4
CAS#:21913-98-4