Tau Peptide (337-368) (Repeat 4 Domain) trifluoroacetate salt
Modify Date: 2025-09-14 13:41:55
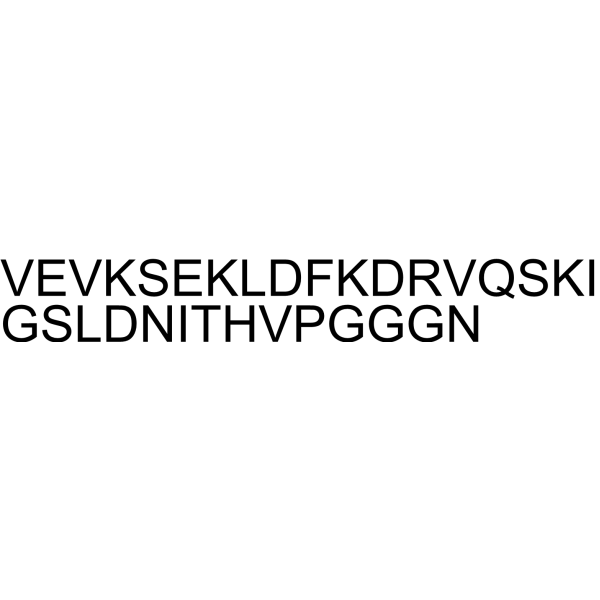
Tau Peptide (337-368) (Repeat 4 Domain) trifluoroacetate salt structure
|
Common Name | Tau Peptide (337-368) (Repeat 4 Domain) trifluoroacetate salt | ||
|---|---|---|---|---|
| CAS Number | 330456-27-4 | Molecular Weight | 3467.84 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C150H248N44O50 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Tau Peptide (337-368) (Repeat 4 Domain) trifluoroacetate saltTau Peptide (337-368) (Repeat 4 Domain) is a polypeptide that can be found by peptide screening. Peptide screening is a research tool that pools active peptides primarily by immunoassay. Peptide screening can be used for protein interaction, functional analysis, epitope screening, especially in the field of agent research and development[1]. |
| Name | L-Valyl-L-α-glutamyl-L-valyl-L-lysyl-L-seryl-L-α-glutamyl-L-lysyl-L-leucyl-L-α-aspartyl-L-phenylalanyl-L-lysyl-L-α-aspartyl-L-arginyl-L-valyl-L-glutaminyl-L-seryl-L-lysyl-L-isoleucylglycyl-L-seryl-L-leucyl-L-α-aspartyl-L-asparaginyl-L-isoleucyl-L-threonyl-L-histidyl-L-valyl-L-prolylglycylglycylglycyl-L-asparagine |
|---|---|
| Synonym | More Synonyms |
| Description | Tau Peptide (337-368) (Repeat 4 Domain) is a polypeptide that can be found by peptide screening. Peptide screening is a research tool that pools active peptides primarily by immunoassay. Peptide screening can be used for protein interaction, functional analysis, epitope screening, especially in the field of agent research and development[1]. |
|---|---|
| Related Catalog | |
| References |
[1]. Birnbaum S, et al. Peptide screening. Current Opinion in Biotechnology, 1992, 3(1): 49-54. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C150H248N44O50 |
| Molecular Weight | 3467.84 |
| Exact Mass | 3465.821533 |
| LogP | -6.65 |
| Index of Refraction | 1.654 |
| InChIKey | RTQDZXJNEXPLFB-NBLZHOLVSA-N |
| SMILES | CCC(C)C(NC(=O)C(CCCCN)NC(=O)C(CO)NC(=O)C(CCC(N)=O)NC(=O)C(NC(=O)C(CCCNC(=N)N)NC(=O)C(CC(=O)O)NC(=O)C(CCCCN)NC(=O)C(Cc1ccccc1)NC(=O)C(CC(=O)O)NC(=O)C(CC(C)C)NC(=O)C(CCCCN)NC(=O)C(CCC(=O)O)NC(=O)C(CO)NC(=O)C(CCCCN)NC(=O)C(NC(=O)C(CCC(=O)O)NC(=O)C(N)C(C)C)C(C)C)C(C)C)C(=O)NCC(=O)NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC(=O)O)C(=O)NC(CC(N)=O)C(=O)NC(C(=O)NC(C(=O)NC(Cc1cnc[nH]1)C(=O)NC(C(=O)N1CCCC1C(=O)NCC(=O)NCC(=O)NCC(=O)NC(CC(N)=O)C(=O)O)C(C)C)C(C)O)C(C)CC.O=C(O)C(F)(F)F |
| L-Asparagine, L-valyl-L-α-glutamyl-L-valyl-L-lysyl-L-seryl-L-α-glutamyl-L-lysyl-L-leucyl-L-α-aspartyl-L-phenylalanyl-L-lysyl-L-α-aspartyl-L-arginyl-L-valyl-L-glutaminyl-L-seryl-L-lysyl 
-L-isoleucylglycyl-L-seryl-L-leucyl-L-α-aspartyl-L-asparaginyl-L-isoleucyl-L-threonyl-L-histidyl-L-valyl-L-prolylglycylglycylglycyl- |
| L-Valyl-L-α-glutamyl-L-valyl-L-lysyl-L-seryl-L-α-glutamyl-L-lysyl-L-leucyl-L-α-aspartyl-L-phenylalanyl-L-lysyl-L-α-aspartyl-L-arginyl-L-valyl-L-glutaminyl-L-seryl-L-lysyl-L-isoleucylgl 
ycyl-L-seryl-L-leucyl-L-α-aspartyl-L-asparaginyl-L-isoleucyl-L-threonyl-L-histidyl-L-valyl-L-prolylglycylglycylglycyl-L-asparagine |