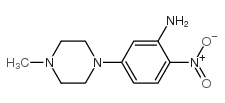
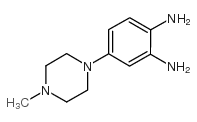
Hoechst 33258 analog 5
Modify Date: 2024-01-03 00:25:26
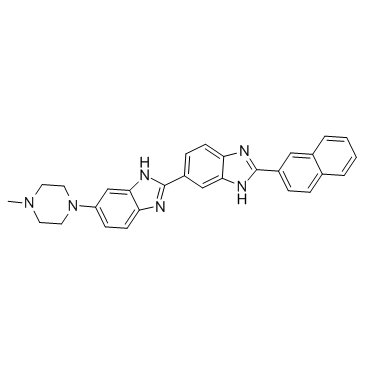
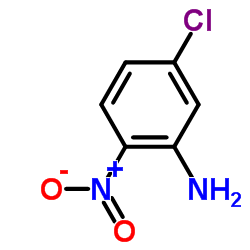
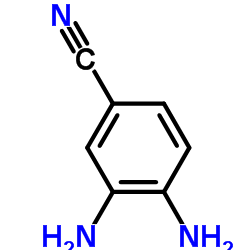
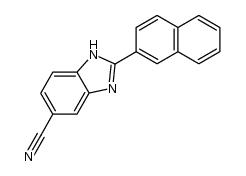
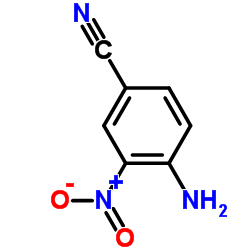
Hoechst 33258 analog 5 structure
|
Common Name | Hoechst 33258 analog 5 | ||
|---|---|---|---|---|
| CAS Number | 23491-55-6 | Molecular Weight | 458.55700 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C29H26N6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Hoechst 33258 analog 5Hoechst 33258 analog 5 is a analog of Hoechst stains, which are part of a family of blue fluorescent dyes used to stain DNA.IC50 Value:Target: These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C.The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably.Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. |
| Name | 6-(4-methylpiperazin-1-yl)-2-(2-naphthalen-2-yl-3H-benzimidazol-5-yl)-1H-benzimidazole |
|---|---|
| Synonym | More Synonyms |
| Description | Hoechst 33258 analog 5 is a analog of Hoechst stains, which are part of a family of blue fluorescent dyes used to stain DNA.IC50 Value:Target: These Bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similarexcitation/emission spectra. Both dyes are excited by ultraviolet light at around 350 nm, and both emit blue/cyan fluorescent light around anemission maximum at 461 nm. Unbound dye has its maximum fluorescence emission in the 510-540 nm range. Hoechst dyes are soluble in water and in organic solvents such as dimethyl formamide or dimethyl sulfoxide. Concentrations can be achieved of up to 10 mg/mL. Aqueous solutions are stable at 2-6 °C for at least six months when protected from light. For long-term storage the solutions are instead frozen at ≤-20 °C.The dyes bind to the minor groove of double-stranded DNA with a preference for sequences rich in adenine andthymine. Although the dyes can bind to all nucleic acids, AT-rich double-stranded DNA strands enhance fluorescence considerably.Hoechst dyes are cell-permeable and can bind to DNA in live or fixed cells. Therefore, these stains are often called supravital, which means that cells survive a treatment with these compounds. Cells that express specific ATP-binding cassette transporter proteins can also actively transport these stains out of their cytoplasm. |
|---|---|
| Related Catalog | |
| References |
[2]. a b c "Hoechst Stains". Invitrogren (Molecular Probes). |
| Molecular Formula | C29H26N6 |
|---|---|
| Molecular Weight | 458.55700 |
| Exact Mass | 458.22200 |
| PSA | 63.84000 |
| LogP | 5.68110 |
| Storage condition | -20℃ |
|
~% 
Hoechst 33258 a... CAS#:23491-55-6 |
| Literature: Jung Sun Kim; Gatto; Yu; Liu; LaVoie Journal of Medicinal Chemistry, 1996 , vol. 39, # 4 p. 992 - 998 |
|
~% 
Hoechst 33258 a... CAS#:23491-55-6 |
| Literature: Jung Sun Kim; Gatto; Yu; Liu; LaVoie Journal of Medicinal Chemistry, 1996 , vol. 39, # 4 p. 992 - 998 |
|
~% 
Hoechst 33258 a... CAS#:23491-55-6 |
| Literature: Jung Sun Kim; Gatto; Yu; Liu; LaVoie Journal of Medicinal Chemistry, 1996 , vol. 39, # 4 p. 992 - 998 |
|
~% 
Hoechst 33258 a... CAS#:23491-55-6 |
| Literature: Jung Sun Kim; Gatto; Yu; Liu; LaVoie Journal of Medicinal Chemistry, 1996 , vol. 39, # 4 p. 992 - 998 |
|
~% 
Hoechst 33258 a... CAS#:23491-55-6 |
| Literature: Jung Sun Kim; Gatto; Yu; Liu; LaVoie Journal of Medicinal Chemistry, 1996 , vol. 39, # 4 p. 992 - 998 |
|
~% 
Hoechst 33258 a... CAS#:23491-55-6 |
| Literature: Jung Sun Kim; Gatto; Yu; Liu; LaVoie Journal of Medicinal Chemistry, 1996 , vol. 39, # 4 p. 992 - 998 |
|
~17% 
Hoechst 33258 a... CAS#:23491-55-6 |
| Literature: Jung Sun Kim; Gatto; Yu; Liu; LaVoie Journal of Medicinal Chemistry, 1996 , vol. 39, # 4 p. 992 - 998 |
| cs-1316 |
| Hoechst 33258 analog 5 |