Taltobulin trifluoroacetate
Modify Date: 2025-09-18 19:51:41
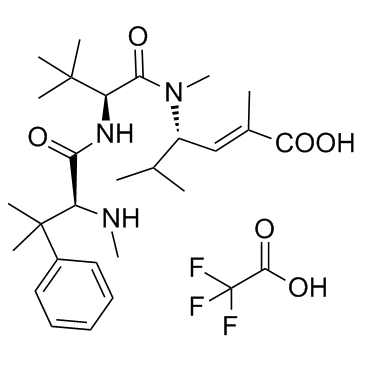
Taltobulin trifluoroacetate structure
|
Common Name | Taltobulin trifluoroacetate | ||
|---|---|---|---|---|
| CAS Number | 228266-41-9 | Molecular Weight | 587.671 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C29H44F3N3O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Taltobulin trifluoroacetateTaltobulin trifluoroacetate (HTI-286; SPA-110) is an analogue of Hemiasterlin; potent tubulin inhibitor; ADCs cytotoxin.IC50 value:Target: tubulinin vitro: HTI-286 significantly inhibited proliferation of all three hepatic tumor cell lines (mean IC50 = 2 nmol/L +/- 1 nmol/L) in vitro. Interestingly, no decrease in viable primary human hepatocytes (PHH) was detected under HTI-286 exposure [1]. In all cell lines tested, HTI-286 was a potent inhibitor of proliferation and induced marked increases in apoptosis. Despite similar transcriptomic changes regarding cell death and cell cycle regulating genes after exposure to HTI-286 or docetaxel, array analysis revealed distinct molecular signatures for both compounds [2].in vivo: Intravenous administration of HTI-286 significantly inhibited tumor growth in vivo (rat allograft model) [1]. HTI-286 significantly inhibited growth of PC-3 and LNCaP xenografts and retained potency in PC-3dR tumors. Simultaneous castration plus HTI-286 therapy was superior to sequential treatment in the LNCaP model [2]. |
| Name | Taltobulin trifluoroacetate |
|---|---|
| Synonym | More Synonyms |
| Description | Taltobulin trifluoroacetate (HTI-286; SPA-110) is an analogue of Hemiasterlin; potent tubulin inhibitor; ADCs cytotoxin.IC50 value:Target: tubulinin vitro: HTI-286 significantly inhibited proliferation of all three hepatic tumor cell lines (mean IC50 = 2 nmol/L +/- 1 nmol/L) in vitro. Interestingly, no decrease in viable primary human hepatocytes (PHH) was detected under HTI-286 exposure [1]. In all cell lines tested, HTI-286 was a potent inhibitor of proliferation and induced marked increases in apoptosis. Despite similar transcriptomic changes regarding cell death and cell cycle regulating genes after exposure to HTI-286 or docetaxel, array analysis revealed distinct molecular signatures for both compounds [2].in vivo: Intravenous administration of HTI-286 significantly inhibited tumor growth in vivo (rat allograft model) [1]. HTI-286 significantly inhibited growth of PC-3 and LNCaP xenografts and retained potency in PC-3dR tumors. Simultaneous castration plus HTI-286 therapy was superior to sequential treatment in the LNCaP model [2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C29H44F3N3O6 |
|---|---|
| Molecular Weight | 587.671 |
| Exact Mass | 587.318237 |
| InChIKey | SHWPCKJNFBDPFA-LPWSJWOVSA-N |
| SMILES | CNC(C(=O)NC(C(=O)N(C)C(C=C(C)C(=O)O)C(C)C)C(C)(C)C)C(C)(C)c1ccccc1.O=C(O)C(F)(F)F |
| Storage condition | 2-8℃ |
| N,β,β-Trimethyl-L-phenylalanyl-N-[(3S,4E)-5-carboxy-2-methyl-4-hexen-3-yl]-N,3-dimethylvalinamide trifluoroacetate (1:1) |
| Acetic acid, 2,2,2-trifluoro-, compd. with valinamide, N,β,β-trimethyl-L-phenylalanyl-N-[(1S,2E)-3-carboxy-1-(1-methylethyl)-2-buten-1-yl]-N,3-dimethyl- (1:1) |
| Taltobulin (trifluoroacetate) |