4-(1H-Imidazol-1-yl)aniline
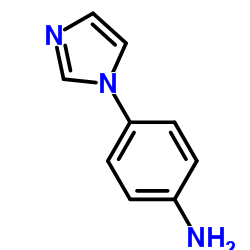
4-(1H-Imidazol-1-yl)aniline structure
|
Common Name | 4-(1H-Imidazol-1-yl)aniline | ||
|---|---|---|---|---|
| CAS Number | 2221-00-3 | Molecular Weight | 159.188 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 355.4±25.0 °C at 760 mmHg | |
| Molecular Formula | C9H9N3 | Melting Point | 143-147 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 168.7±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 4-imidazol-1-ylaniline |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 355.4±25.0 °C at 760 mmHg |
| Melting Point | 143-147 °C(lit.) |
| Molecular Formula | C9H9N3 |
| Molecular Weight | 159.188 |
| Flash Point | 168.7±23.2 °C |
| Exact Mass | 159.079651 |
| PSA | 43.84000 |
| LogP | 0.70 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.641 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933290090 |
| Precursor 7 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Improved purification of immunoglobulin G from plasma by mixed-mode chromatography.
J. Sep. Sci. 37(23) , 3461-72, (2014) Efficient loading of immunoglobulin G in mixed-mode chromatography is often a serious bottleneck in the chromatographic purification of immunoglobulin G. In this work, a mixed-mode ligand, 4-(1H-imida... |
|
|
Effect of dextran layer on protein uptake to dextran-grafted adsorbents for ion-exchange and mixed-mode chromatography.
J. Sep. Sci. 34(21) , 2950-9, (2011) In the current research, a series of dextran-grafted adsorbents were prepared using sulfopropyl and 4-(1H-imidazol-1-yl) aniline as chromatographic ligands for ion-exchange (IEC) and mixed-mode chroma... |
|
|
4-(1H-imidazol-1-yl) aniline: a new ligand of mixed-mode chromatography for antibody purification.
J. Chromatogr. A. 1216(33) , 6081-7, (2009) 4-(1H-imidazol-1-yl) aniline (AN) was immobilized on Sepharose CL-6B (AN-Sepharose) for use as a new ligand of mixed-mode chromatography. Adsorption equilibria of immunoglobulin G (IgG) and bovine ser... |
| 4-(1H-Imidazole-1-yl)aniline |
| 4-(1H-imidazol-1-yl)phenylamine |
| 4-imidazolylphenylamine |
| 4-(1H-Imidazol-1-yl)aniline |
| MFCD01074865 |
| Benzenamine, 4-(1H-imidazol-1-yl)- |
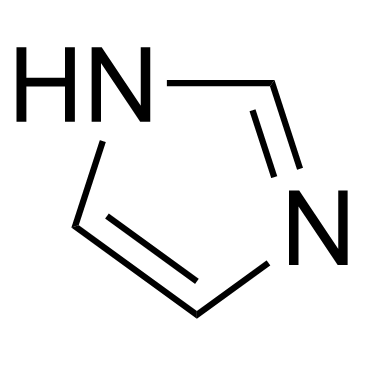 CAS#:288-32-4
CAS#:288-32-4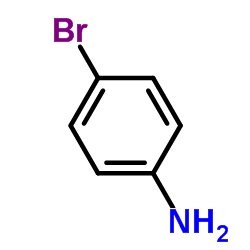 CAS#:106-40-1
CAS#:106-40-1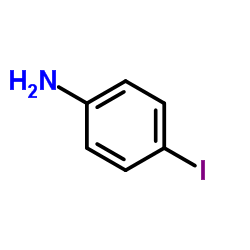 CAS#:540-37-4
CAS#:540-37-4 CAS#:2301-25-9
CAS#:2301-25-9 CAS#:51581-54-5
CAS#:51581-54-5 CAS#:7772-99-8
CAS#:7772-99-8 CAS#:350-46-9
CAS#:350-46-9 CAS#:647835-36-7
CAS#:647835-36-7 CAS#:647835-37-8
CAS#:647835-37-8 CAS#:647835-45-8
CAS#:647835-45-8
