I-CBP112 hydrochloride
Modify Date: 2025-08-28 13:17:16
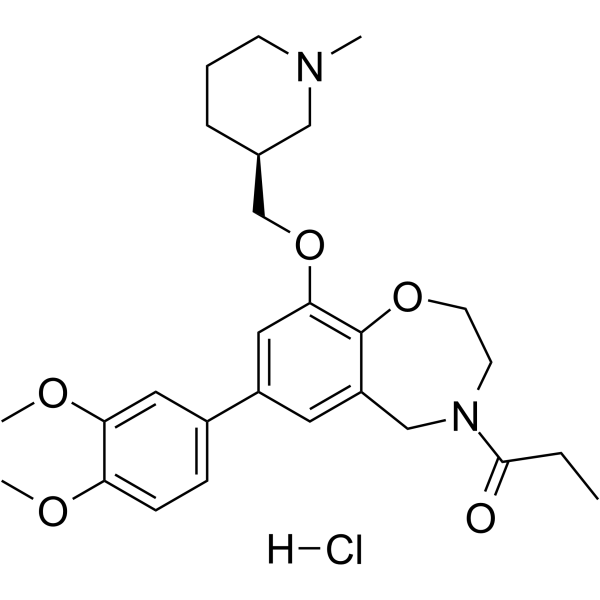
I-CBP112 hydrochloride structure
|
Common Name | I-CBP112 hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2147701-33-3 | Molecular Weight | 505.046 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H37ClN2O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of I-CBP112 hydrochlorideI-CBP112 hydrochloride is a selective inhibitor of CBP/P300 that directly binds their bromodomains (Kds = 142 and 625 nM, respectively). I-CBP112 significantly reduces the leukemia-initiating potential of MLL-AF9(+) acute myeloid leukemia cells in a dose-dependent manner in vitro and in vivo. I-CBP112 increases the cytotoxic activity of BET bromodomain inhibitor JQ1 as well as doxorubicin[1]. |
| Name | I-CBP112 (hydrochloride) |
|---|---|
| Synonym | More Synonyms |
| Description | I-CBP112 hydrochloride is a selective inhibitor of CBP/P300 that directly binds their bromodomains (Kds = 142 and 625 nM, respectively). I-CBP112 significantly reduces the leukemia-initiating potential of MLL-AF9(+) acute myeloid leukemia cells in a dose-dependent manner in vitro and in vivo. I-CBP112 increases the cytotoxic activity of BET bromodomain inhibitor JQ1 as well as doxorubicin[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C27H37ClN2O5 |
|---|---|
| Molecular Weight | 505.046 |
| Exact Mass | 504.239105 |
| I-CBP112 (hydrochloride) |
| 1-[7-(3,4-Dimethoxyphenyl)-9-{[(3S)-1-methyl-3-piperidinyl]methoxy}-2,3-dihydro-1,4-benzoxazepin-4(5H)-yl]-1-propanone hydrochloride (1:1) |
| 1-Propanone, 1-[7-(3,4-dimethoxyphenyl)-2,3-dihydro-9-[[(3S)-1-methyl-3-piperidinyl]methoxy]-1,4-benzoxazepin-4(5H)-yl]-, hydrochloride (1:1) |