JF669, SE
Modify Date: 2025-11-11 17:37:55
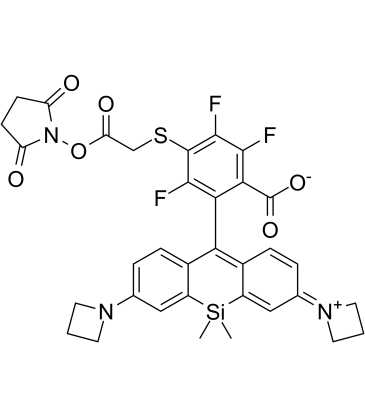
JF669, SE structure
|
Common Name | JF669, SE | ||
|---|---|---|---|---|
| CAS Number | 2127150-20-1 | Molecular Weight | 693.76 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C34H30F3N3O6SSi | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of JF669, SEJF669, SE, a red fluorescent dye, can be directly reacted with the available thiol-containing HaloTag ligand under mild conditions (DIEA, DMF) to afford a JF669−HaloTag ligand in a single step (Ex = 669 nm; Em = 682 nm)[1]. |
| Name | JF669, SE |
|---|
| Description | JF669, SE, a red fluorescent dye, can be directly reacted with the available thiol-containing HaloTag ligand under mild conditions (DIEA, DMF) to afford a JF669−HaloTag ligand in a single step (Ex = 669 nm; Em = 682 nm)[1]. |
|---|---|
| Related Catalog | |
| In Vitro | JF669 undergoes rapid conversion to thioether product (<5 min to completion) in organic solvents, where the dye predominantly adopts the closed form[1]. JF669 displays comparatively excellent photostability, retaining 97% fluorescence after the same number of cycles[1]. |
| References |
| Molecular Formula | C34H30F3N3O6SSi |
|---|---|
| Molecular Weight | 693.76 |