NVP DPP 728 dihydrochloride
Modify Date: 2024-01-28 11:16:26
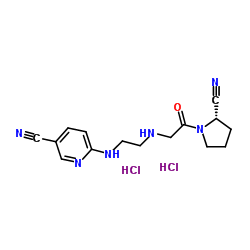
NVP DPP 728 dihydrochloride structure
|
Common Name | NVP DPP 728 dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 207556-62-5 | Molecular Weight | 371.265 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H20Cl2N6O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of NVP DPP 728 dihydrochlorideNVP-DPP728 dihydrochloride is a potent, selective and orally active dipeptidyl peptidase IV (DPP-IV) inhibitor with a Ki of 11 nM. NVP-DPP728 dihydrochloride can be used for the research of diabetes mellitus[1][2]. |
| Name | 6-{[2-({2-[(2S)-2-Cyano-1-pyrrolidinyl]-2-oxoethyl}amino)ethyl]am ino}nicotinonitrile dihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | NVP-DPP728 dihydrochloride is a potent, selective and orally active dipeptidyl peptidase IV (DPP-IV) inhibitor with a Ki of 11 nM. NVP-DPP728 dihydrochloride can be used for the research of diabetes mellitus[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 11 nM (DPP-IV) [1] |
| In Vivo | NVP-DPP728 inhibits DPP-IV and improves insulin secretion and glucose tolerance, probably through augmentation of the effects of endogenous GLP-1[2]. Animal Model: Obese (fa/fa) and lean (FA/?) Zucker rats[2] Dosage: 3.715 mg/kg Administration: Oral administration Result: Led to inhibition of plasma DPP-IV activity. |
| References |
| Molecular Formula | C15H20Cl2N6O |
|---|---|
| Molecular Weight | 371.265 |
| Exact Mass | 370.107574 |
| PSA | 108.07000 |
| LogP | 1.82406 |
| 3-Pyridinecarbonitrile, 6-[[2-[[2-[(2S)-2-cyano-1-pyrrolidinyl]-2-oxoethyl]amino]ethyl]amino]-, hydrochloride (1:2) |
| 6-{[2-({2-[(2S)-2-Cyano-1-pyrrolidinyl]-2-oxoethyl}amino)ethyl]amino}nicotinonitrile dihydrochloride |
| 6-{[2-({2-[(2S)-2-Cyanopyrrolidin-1-yl]-2-oxoethyl}amino)ethyl]amino}nicotinonitrile dihydrochloride |