2-(1,3-thiazol-2-yldisulfanyl)-1,3-thiazole
Modify Date: 2025-08-25 10:42:59
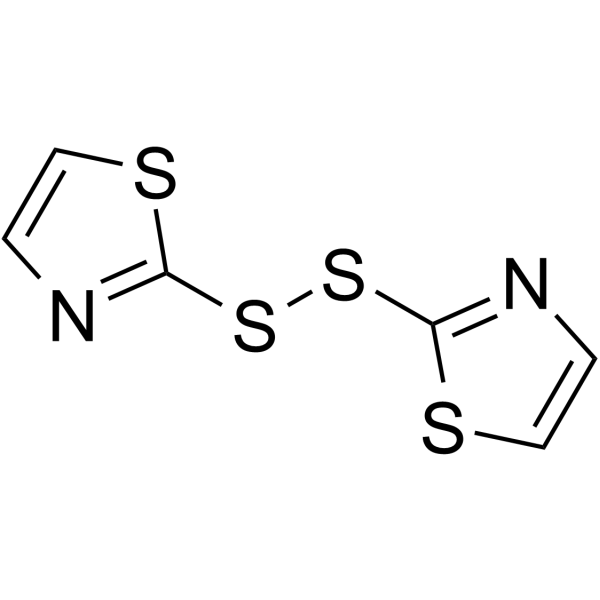
2-(1,3-thiazol-2-yldisulfanyl)-1,3-thiazole structure
|
Common Name | 2-(1,3-thiazol-2-yldisulfanyl)-1,3-thiazole | ||
|---|---|---|---|---|
| CAS Number | 20362-54-3 | Molecular Weight | 232.36900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H4N2S4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of 2-(1,3-thiazol-2-yldisulfanyl)-1,3-thiazoleFBPase-IN-1 is a potent FBPase (Fructose-1,6-bisphosphatase) inhibitor for Type 2 diabetes (T2D) study with an IC50 of 0.22 μM. FBPase-IN-1 can reduce blood glucose levels and ameliorate glucose tolerance. FBPase-IN-1 modifies the C128 site, regulates the N125-S124-S123 allosteric pathway of FBPase and affects the catalytic activity of FBPase[1]. |
| Name | 2-(1,3-thiazol-2-yldisulfanyl)-1,3-thiazole |
|---|---|
| Synonym | More Synonyms |
| Description | FBPase-IN-1 is a potent FBPase (Fructose-1,6-bisphosphatase) inhibitor for Type 2 diabetes (T2D) study with an IC50 of 0.22 μM. FBPase-IN-1 can reduce blood glucose levels and ameliorate glucose tolerance. FBPase-IN-1 modifies the C128 site, regulates the N125-S124-S123 allosteric pathway of FBPase and affects the catalytic activity of FBPase[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.22 μM (FBPase)[1] |
| In Vitro | FBPase-IN-1 (compund 3a) (1.5625-200 μM, 48 hours) shows a low cytotoxicity with an IC50 of 75.08 μM[1]. FBPase-IN-1 (0-45 min) inhibits FBPase in a time-dependent manner, with IC50 decreasing from 0.28 to 0.09 μM, shows FBPase-IN-1 is an irreversible covalent inhibitor of FBPase[1]. |
| In Vivo | FBPase-IN-1 (12 h-fasted ICR mouse, n = 6, male, 25 mg/kg, IP) shows good FBPase inhibitory activity (IC50 = 0.22 μM), satisfying glucose-lowering effect in vivo (54% reduction in blood glucose at 25 mg/kg)[1]. FBPase-IN-1 (6 or 7 weeks, ICR mice, n = 6, male, 25 mg/kg, IP for 4 hours, once) reduces blood glucose levels by targeting FBPase and inhibiting the GNG process[1]. Animal Model: ICR mouse (12 h-fasted, n = 6, male)[1] Dosage: 25 mg/kg Administration: IP, once Result: Lowered glucose. Animal Model: ICR mice (6 or 7 weeks, n = 6, male)[1] Dosage: 25 mg/kg Administration: IP, for 4 h, once Result: Reduced blood glucose levels by targeting FBPase and inhibiting the GNG process. |
| References |
| Molecular Formula | C6H4N2S4 |
|---|---|
| Molecular Weight | 232.36900 |
| Exact Mass | 231.92600 |
| PSA | 132.86000 |
| LogP | 3.39900 |
| HS Code | 2934100090 |
|---|
|
~79% 
2-(1,3-thiazol-... CAS#:20362-54-3 |
| Literature: Abdel-Mohsen, Heba T.; Sudheendran, Kavitha; Conrad, Juergen; Beifuss, Uwe Green Chemistry, 2013 , vol. 15, # 6 p. 1490 - 1495 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934100090 |
|---|---|
| Summary | 2934100090 other compounds containing an unfused thiazole ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
| Thiazole,2,2'-dithiobis |
| 2-thiazol-2-yldisulfanyl-thiazole |
| THI022 |
| Bis-thiazol-2-yl-disulfid |