Lopinavir
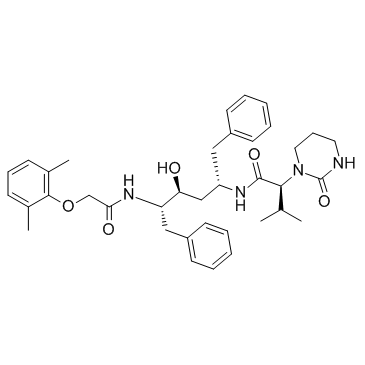
Lopinavir structure
|
Common Name | Lopinavir | ||
|---|---|---|---|---|
| CAS Number | 192725-17-0 | Molecular Weight | 628.801 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 924.2±65.0 °C at 760 mmHg | |
| Molecular Formula | C37H48N4O5 | Melting Point | 124-127°C | |
| MSDS | Chinese USA | Flash Point | 512.7±34.3 °C | |
Use of LopinavirLopinavir is a potent HIV protease inhibitor with Ki of 1.3 pM.Target: HIV proteaseLopinavir is a potent inhibitor of Rh123 efflux in Caco-2 monolayers with IC50 of 1.7 mM. Lopinavir exposure (72 hours) in LS 180V cells reduces the content of intracellular Rh123. Lopinavir induces P-glycoprotein immunoreactive protein and messenger RNA levels in LS 180V cells. Lopinavir inhibits subtype C clone C6 with IC50 of 9.4 nM. Lopinavir inhibits CYP3A with IC50 of 7.3 mM in human liver microsomes, while produces negligible or weak inhibition of human CYP1A2, 2B6, 2C9, 2C19 and 2D6. Lopinavir (10 mg/kg, orally) results in Cmax of 0.8 μg/mL and oral bioavailability of 25% in rats. |
| Name | lopinavir |
|---|---|
| Synonym | More Synonyms |
| Description | Lopinavir is a potent HIV protease inhibitor with Ki of 1.3 pM.Target: HIV proteaseLopinavir is a potent inhibitor of Rh123 efflux in Caco-2 monolayers with IC50 of 1.7 mM. Lopinavir exposure (72 hours) in LS 180V cells reduces the content of intracellular Rh123. Lopinavir induces P-glycoprotein immunoreactive protein and messenger RNA levels in LS 180V cells. Lopinavir inhibits subtype C clone C6 with IC50 of 9.4 nM. Lopinavir inhibits CYP3A with IC50 of 7.3 mM in human liver microsomes, while produces negligible or weak inhibition of human CYP1A2, 2B6, 2C9, 2C19 and 2D6. Lopinavir (10 mg/kg, orally) results in Cmax of 0.8 μg/mL and oral bioavailability of 25% in rats. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 924.2±65.0 °C at 760 mmHg |
| Melting Point | 124-127°C |
| Molecular Formula | C37H48N4O5 |
| Molecular Weight | 628.801 |
| Flash Point | 512.7±34.3 °C |
| Exact Mass | 628.362488 |
| PSA | 120.00000 |
| LogP | 6.26 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.577 |
| Storage condition | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| Stability | Hygroscopic |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
|
ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease.
Antimicrob. Agents Chemother. 42(12) , 3218-24, (1998) The valine at position 82 (Val 82) in the active site of the human immunodeficiency virus (HIV) protease mutates in response to therapy with the protease inhibitor ritonavir. By using the X-ray crysta... |
|
|
HIV-1 subtype influences susceptibility and response to monotherapy with the protease inhibitor lopinavir/ritonavir.
J. Antimicrob. Chemother. 70(1) , 243-8, (2015) PI susceptibility results from a complex interplay between protease and Gag proteins, with Gag showing wide variation across HIV-1 subtypes. We explored the impact of pre-treatment susceptibility on t... |
|
|
Antiretroviral therapy response among HIV-2 infected patients: a systematic review.
BMC Infect. Dis. 14 , 461, (2014) Few data are available on antiretroviral therapy (ART) response among HIV-2 infected patients. We conducted a systematic review on treatment outcomes among HIV-2 infected patients on ART, focusing on ... |
| 1(2H)-Pyrimidineacetamide, N-[(1S,3S,4S)-4-[[2-(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-α-(1-methylethyl)-2-oxo-, (αS)- |
| (2S)-N-[(2S,4S,5S)-5-{[(2,6-Dimethylphenoxy)acetyl]amino}-4-hydroxy-1,6-diphenyl-2-hexanyl]-3-methyl-2-(2-oxotetrahydro-1(2H)-pyrimidinyl)butanamide |
| (2S)-N-[(1S,3S,4S)-1-benzyl-4-{[(2,6-diméthylphénoxy)acétyl]amino}-3-hydroxy-5-phénylpentyl]-3-méthyl-2-(2-oxotétrahydropyrimidin-1(2H)-yl)butanamide |
| ABT 378 |
| (2S)-N-[(1S,3S,4S)-1-Benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-hydroxy-5-phenylpentyl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamid |
| Lopinavir |
| Aluviran |
| 1(2H)-pyrimidineacetamide, N-[(1S,3S,4S)-4-[[(2,6-dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-α-(1-methylethyl)-2-oxo-, (αS)- |
| (aS)-Tetrahydro-N-((aS)-a-((2S,3S)-2-hydroxy-4-phenyl-3-(2-(2,6-xylyloxy)acetamido)butyl)phenethyl)-a-isopropyl-2-oxo-1(2H)-pyrimidineacetamide |
| Koletr |
| (2S)-N-[(1S,3S,4S)-1-benzyl-4-{[(2,6-dimethylphenoxy)acetyl]amino}-3-hydroxy-5-phenylpentyl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamide |
| (2S)-N-[(2S,4S,5S)-5-{[(2,6-Dimethylphenoxy)acetyl]amino}-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamide |
| ABT 378/r |
| Koletra |
| MFCD04973616 |
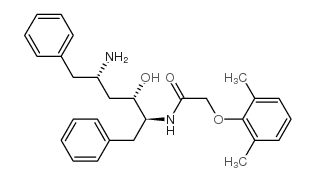 CAS#:192725-49-8
CAS#:192725-49-8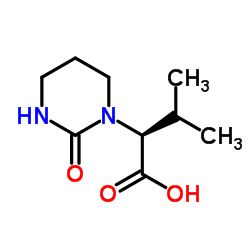 CAS#:192725-50-1
CAS#:192725-50-1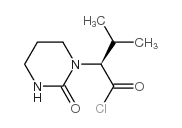 CAS#:192800-77-4
CAS#:192800-77-4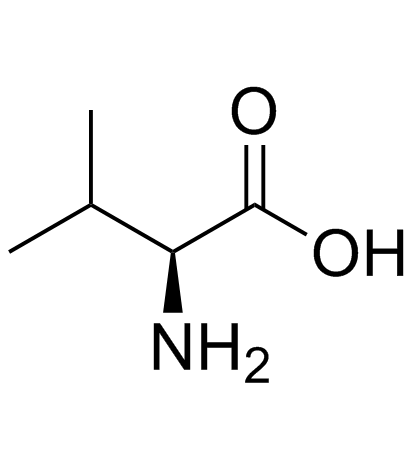 CAS#:72-18-4
CAS#:72-18-4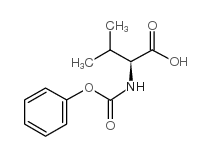 CAS#:126147-70-4
CAS#:126147-70-4![[(1S,3S,4S)-4-Amino-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]carbamic acid 1,1-dimethylethyl ester Structure](https://image.chemsrc.com/caspic/437/144163-85-9.png) CAS#:144163-85-9
CAS#:144163-85-9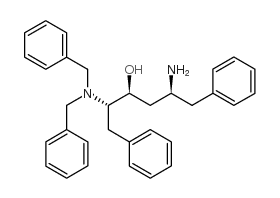 CAS#:156732-15-9
CAS#:156732-15-9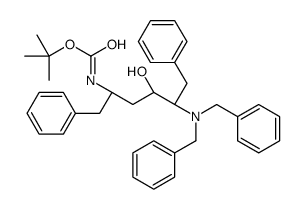 CAS#:162849-93-6
CAS#:162849-93-6
