tert-Butyl [(4-methylphenyl)sulfonyl]carbamate
![tert-Butyl [(4-methylphenyl)sulfonyl]carbamate Structure](https://image.chemsrc.com/caspic/152/18303-04-3.png)
tert-Butyl [(4-methylphenyl)sulfonyl]carbamate structure
|
Common Name | tert-Butyl [(4-methylphenyl)sulfonyl]carbamate | ||
|---|---|---|---|---|
| CAS Number | 18303-04-3 | Molecular Weight | 271.333 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C12H17NO4S | Melting Point | 121-123ºC(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Name | tert-butyl N-(4-methylphenyl)sulfonylcarbamate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Melting Point | 121-123ºC(lit.) |
| Molecular Formula | C12H17NO4S |
| Molecular Weight | 271.333 |
| Exact Mass | 271.087830 |
| PSA | 80.85000 |
| LogP | 2.56 |
| Index of Refraction | 1.522 |
| Water Solubility | chloroform: soluble25mg/mL, clear, colorless |
| Safety Phrases | 24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2935009090 |
|
~96% ![tert-Butyl [(4-methylphenyl)sulfonyl]carbamate Structure](https://image.chemsrc.com/caspic/152/18303-04-3.png)
tert-Butyl [(4-... CAS#:18303-04-3 |
| Literature: Nouria, Azita; Akbari, Jafar; Heydaric, Akbar; Nouri, Arezu Letters in Organic Chemistry, 2011 , vol. 8, # 1 p. 38 - 42 |
|
~96% ![tert-Butyl [(4-methylphenyl)sulfonyl]carbamate Structure](https://image.chemsrc.com/caspic/152/18303-04-3.png)
tert-Butyl [(4-... CAS#:18303-04-3 |
| Literature: Moore, Joel D; Harned, Andrew M; Henle, Julia; Flynn, Daniel L; Hanson, Paul R Organic letters, 2002 , vol. 4, # 11 p. 1847 - 1849 |
|
~95% ![tert-Butyl [(4-methylphenyl)sulfonyl]carbamate Structure](https://image.chemsrc.com/caspic/152/18303-04-3.png)
tert-Butyl [(4-... CAS#:18303-04-3 |
| Literature: Johnson II, David C.; Widlanski, Theodore S. Tetrahedron Letters, 2004 , vol. 45, # 46 p. 8483 - 8487 |
|
~% ![tert-Butyl [(4-methylphenyl)sulfonyl]carbamate Structure](https://image.chemsrc.com/caspic/152/18303-04-3.png)
tert-Butyl [(4-... CAS#:18303-04-3 |
| Literature: Tetrahedron Letters, , vol. 45, # 46 p. 8483 - 8487 |
|
~% ![tert-Butyl [(4-methylphenyl)sulfonyl]carbamate Structure](https://image.chemsrc.com/caspic/152/18303-04-3.png)
tert-Butyl [(4-... CAS#:18303-04-3 |
| Literature: Tetrahedron Letters, , vol. 45, # 46 p. 8483 - 8487 |
|
~% ![tert-Butyl [(4-methylphenyl)sulfonyl]carbamate Structure](https://image.chemsrc.com/caspic/152/18303-04-3.png)
tert-Butyl [(4-... CAS#:18303-04-3 |
| Literature: Tetrahedron Letters, , vol. 45, # 46 p. 8483 - 8487 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Tetrahedron Lett. 30 , 5709, (1989)
|
|
|
Use of the Mitsunobu reaction in the synthesis of orthogonally protected a, ß-diaminopropionic acids. Kelleher F.
Tetrahedron Lett. 48(28) , 4879-82, (2007)
|
|
|
Diastereoselective formal synthesis of a monoterpene alkaloid, (-)-incarvilline.
J. Org. Chem. 72(17) , 6541-7, (2007) Diastereoselective formal synthesis of a monoterpene alkaloid, (-)-incarvilline, the key intermediate for the synthesis of (-)-incarvillateine, was achieved by using an intramolecular Pauson-Khand rea... |
| Weinreb's reagent |
| tert-butyl tosylcarbamate |
| N-Boc p-toluenesufonamide |
| tert-Butyl [(4-methylphenyl)sulfonyl]carbamate |
| N-Boc-N-tosylamide |
| 2-Methyl-2-propanyl [(4-methylphenyl)sulfonyl]carbamate |
| N-(tert-Butoxycarbonyl)-p-toluenesulfonaMide |
| N-Boc-p-toluenesulfonamide |
| Carbamic acid, N-[(4-methylphenyl)sulfonyl]-, 1,1-dimethylethyl ester |
| N-tosyl-N-Boc amide |
| N-Boc-N-tosylamine |
| MFCD00134267 |


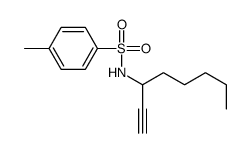 CAS#:139372-08-0
CAS#:139372-08-0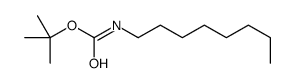 CAS#:101506-39-2
CAS#:101506-39-2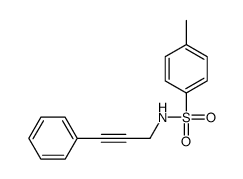 CAS#:305837-95-0
CAS#:305837-95-0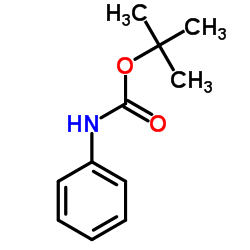 CAS#:3422-01-3
CAS#:3422-01-3 CAS#:133886-40-5
CAS#:133886-40-5 CAS#:184357-44-6
CAS#:184357-44-6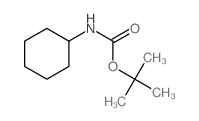 CAS#:3712-40-1
CAS#:3712-40-1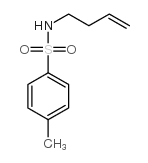 CAS#:10285-80-0
CAS#:10285-80-0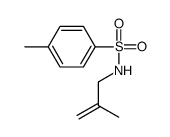 CAS#:1206-41-3
CAS#:1206-41-3
