2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid
Modify Date: 2025-08-25 09:18:01
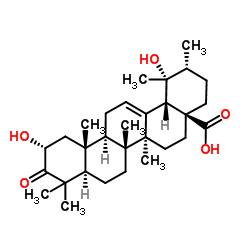
2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid structure
|
Common Name | 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid | ||
|---|---|---|---|---|
| CAS Number | 176983-21-4 | Molecular Weight | 486.683 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 616.9±55.0 °C at 760 mmHg | |
| Molecular Formula | C30H46O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 340.9±28.0 °C | |
Use of 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid, a natural ursane-type triterpene, is a potent inhibitor of HIV Protease (HIV Protease). 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid is also an inhibitor of the activation of Epstein-Barr virus early antigen (EBV-EA). 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid displays an inhibitory activity against nitric oxide production in Lipopolysaccharide (Lipopolysaccharides)-activated RAW 264.7 cells[1][2]. |
| Name | (2α)-2,19-Dihydroxy-3-oxours-12-en-28-oic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid, a natural ursane-type triterpene, is a potent inhibitor of HIV Protease (HIV Protease). 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid is also an inhibitor of the activation of Epstein-Barr virus early antigen (EBV-EA). 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid displays an inhibitory activity against nitric oxide production in Lipopolysaccharide (Lipopolysaccharides)-activated RAW 264.7 cells[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid (0.01-0.1 μM) 在脂多糖激活的巨噬细胞系、RAW 264.7 细胞中显示出对一氧化氮产生的中等抑制活性[1 ]。 |
| In Vivo | 2α,19α-Dihydroxy-3-oxo-urs-12-en-28-oic acid (0.0025%;饮用水;20 周) 显示出对 TPA 诱导的 EBV-EA 激活的抑制作用,并导致显着延迟 小鼠皮肤两阶段癌变[2]。 Animal Model: Female ICR mice (6 weeks old)[2] Dosage: 0.0025% Administration: Drinking water; for 20 weeks Result: Showed an inhibitory effect on the activation of EBV-EA induced by 12-O-tetradecanoylphorbol-13-acetate (TPA). |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 616.9±55.0 °C at 760 mmHg |
| Molecular Formula | C30H46O5 |
| Molecular Weight | 486.683 |
| Flash Point | 340.9±28.0 °C |
| Exact Mass | 486.334534 |
| LogP | 5.45 |
| Vapour Pressure | 0.0±4.0 mmHg at 25°C |
| Index of Refraction | 1.574 |
| InChIKey | LJORXTIGOHMBOS-CMDFFPTJSA-N |
| SMILES | CC1CCC2(C(=O)O)CCC3(C)C(=CCC4C5(C)CC(O)C(=O)C(C)(C)C5CCC43C)C2C1(C)O |
| Storage condition | 2-8℃ |
| 2α,19α-dihydroxy-3-oxo-12-ursen-28-oic acid |
| (2α)-2,19-Dihydroxy-3-oxours-12-en-28-oic acid |
| Urs-12-en-28-oic acid, 2,19-dihydroxy-3-oxo-, (2α)- |