1,3-Dimesitylimidazolidin-1-ium chloride
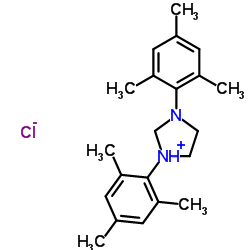
1,3-Dimesitylimidazolidin-1-ium chloride structure
|
Common Name | 1,3-Dimesitylimidazolidin-1-ium chloride | ||
|---|---|---|---|---|
| CAS Number | 173035-10-4 | Molecular Weight | 344.921 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H29ClN2 | Melting Point | 280-286ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1,3-Bis(2,4,6-trimethylphenyl)imidazolinium Chloride |
|---|---|
| Synonym | More Synonyms |
| Melting Point | 280-286ºC |
|---|---|
| Molecular Formula | C21H29ClN2 |
| Molecular Weight | 344.921 |
| Exact Mass | 344.201935 |
| PSA | 6.48000 |
| LogP | 5.75310 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933290090 |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
[(NHC)CuX] complexes: synthesis, characterization and catalytic activities in reduction reactions and Click chemistry. On the advantage of using well-defined catalytic systems.
Dalton Trans. 39 , 7595-7606, (2010) The preparation of three series of [(NHC)CuX] complexes (NHC = N-heterocyclic carbene, X = Cl, Br, or I) is reported. These syntheses are high yielding and only use readily available starting material... |
|
|
(NHC)Copper(I)-catalyzed [3+2] cycloaddition of azides and mono- or disubstituted alkynes.
Chemistry 12 , 7558, (2006) A versatile and highly efficient catalyst for the Huisgen cycloaddition reaction has been developed. Previously isolated or in situ generated azides yielded 1,2,3-triazoles with differently substitute... |
|
|
Luminescent cyclometalated N-heterocyclic carbene-containing organogold(III) complexes: synthesis, characterization, electrochemistry, and photophysical studies.
J. Am. Chem. Soc. 131 , 9076-9085, (2009) A new class of luminescent mononuclear and dinuclear N-heterocyclic carbene-containing gold(III) complexes has been synthesized and characterized. The X-ray crystal structures of most of the complexes... |
| Imidazolidine, 1,3-bis(2,4,6-trimethylphenyl)-, hydrochloride (1:1) |
| 1,3-Bis(2,4,6-trimethylphenyl)imidazolidinium chloride |
| 1,3-Dimesitylimidazolidin-1-ium chloride |
| 1,3-BIS(2,4,6-TRIMETHYLPHENYL)-IMIDAZOLIDINIUM-CHLORIDE |
| 1,3-Dimesityl-4,5-dihydro-1H-imidazol-3-ium chloride |
| 1,3-Bis(2,4,6-triMethylphenyl)iMidazoliniuM Chloride |
 CAS#:536724-67-1
CAS#:536724-67-1 CAS#:173035-11-5
CAS#:173035-11-5 CAS#:260054-47-5
CAS#:260054-47-5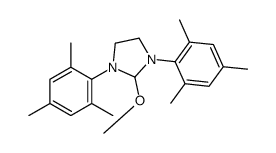 CAS#:465543-01-5
CAS#:465543-01-5