Eganelisib(IPI-549)
Modify Date: 2025-08-22 22:26:25
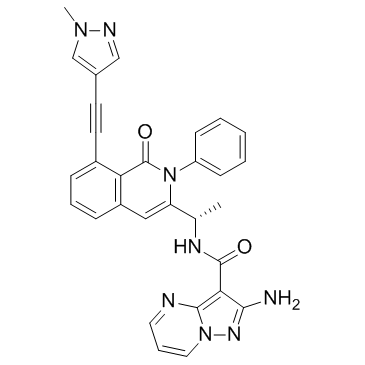
Eganelisib(IPI-549) structure
|
Common Name | Eganelisib(IPI-549) | ||
|---|---|---|---|---|
| CAS Number | 1693758-51-8 | Molecular Weight | 528.576 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C30H24N8O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Eganelisib(IPI-549)IPI549 is a potent and selective PI3Kγ inhibitor with an IC50 of 16 nM. |
| Name | IPI-549 |
|---|---|
| Synonym | More Synonyms |
| Description | IPI549 is a potent and selective PI3Kγ inhibitor with an IC50 of 16 nM. |
|---|---|
| Related Catalog | |
| Target |
PI3Kα:3.2 μM (IC50) PI3Kβ:3.5 μM (IC50) PI3Kγ:16 nM (IC50) |
| In Vitro | IPI-549 inhibits PI3Kγ with IC50 of 16 nM, with >100-fold selectivity over other lipid and protein kinases (PI3Kα IC50=3.2 μM, PI3Kβ IC50=3.5 μM, PI3Kδ IC50>8.4 μM). IPI549 is evaluated for activity across all Class I PI3K isoforms. The binding affinity of IPI549 for PI3K-γ is determined by measuring the individual rates constants and for PI3K-α, β and δ using equilibrium fluorescent titration. IPI549 is found to be a remarkably tight binder to PI3Kγ with a Kd of 290 pM and >58-fold weaker affinity for other Class I PI3K isoforms (PI3Kα Kd=17 nM, PI3Kβ Kd=82 nM, PI3Kδ Kd=23 M). In PI3K-α, -β, -γ, and -δ dependent cellular phospho-AKT assays, IPI549 demonstrates excellent PI3K-γ potency (IC50=1.2 nM) and selectivity against other Class I PI3K isoforms (>146-fold). Cellular IC50s for Class I PI3Kα (250 nM), PI3Kβ (240 nM), PI3Kγ (1.2 nM), PI3Kδ (180 nM) are determined in SKOV-3, 786-O, RAW 264.7, and RAJI cells, respectively, by monitoring inhibition of pAKT S473 by ELISA. Furthermore, IPI549 dose dependently inhibits PI3Kγ dependent bone marrow-derived macrophage (BMDM) migration. IPI549 is also found to be selective against a panel of 80 GPCRs, ion channels, and transporters at 10 μM[1]. |
| In Vivo | IPI-549 demonstrates favorable pharmacokinetic properties and robust inhibition of PI3K-γ mediated neutrophil migration. In vivo (mice, rats, dog, and monkeys), IPI-549 has excellent oral bioavailability, low clearance, and distributed into tissues with a mean volume of distribution of 1.2 L/kg. Overall, IPI-549 has a favorable pharmacokinetic profile to allow potent and selective inhibition of PI3K-γ in vivo. The t1/2 of IPI-549 for mouse, rat, dog and monkey is 3.2, 4.4, 6.7 and 4.3 h, respectively. IPI-549 significantly reduces neutrophil migration in a dose dependent manner in this model when administered orally at all of the tested doses[1]. |
| Animal Admin | C57BL/6J and Balb/c mice (6 to 8 weeks old) are used in this study. On day 0 of the experiments, tumor cells are injected intradermally (i.d.) in the right flank. IPI-549 is administered by oral gavage once a day at 15 mg/kg. Treatment is initiated on day 7 ending on day 21 post tumor implant. Control groups receive vehicle (5% NMP, 95% PEG). Tumors are measured every second or third day with a caliper, and the volume (length×width×height) is calculated. Animals are euthanized for signs of distress or when the total tumor volume reaches 2500 mm3. Finally, Tumors are isolated, and frozen until needed[2]. |
| References |
| Molecular Formula | C30H24N8O2 |
|---|---|
| Molecular Weight | 528.576 |
| Exact Mass | 528.202 |
| Storage condition | -20℃ |
| 2-amino-N-[1-[8-[2-(1-methylpyrazol-4-yl)ethynyl]-1-oxo-2-phenylisoquinolin-3-yl]ethyl]pyrazolo[1,5-a]pyrimidine-3-carboxamide |
| IPI549 |