Fenchol
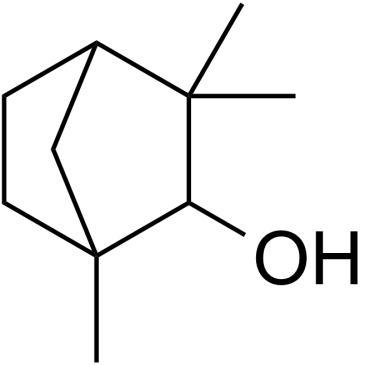
Fenchol structure
|
Common Name | Fenchol | ||
|---|---|---|---|---|
| CAS Number | 1632-73-1 | Molecular Weight | 154.249 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 202.9±8.0 °C at 760 mmHg | |
| Molecular Formula | C10H18O | Melting Point | 35-40ºC | |
| MSDS | Chinese USA | Flash Point | 73.9±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of FencholFenchyl alcohol is a monoterpene alcohol in the essential oils isolated from Douglas fir needles, acts as a fragrance. Fenchyl alcohol strongly inhibits the rumen microbial activity of both sheep and deer[1][2]. |
| Name | (-)-endo-fenchol |
|---|---|
| Synonym | More Synonyms |
| Description | Fenchyl alcohol is a monoterpene alcohol in the essential oils isolated from Douglas fir needles, acts as a fragrance. Fenchyl alcohol strongly inhibits the rumen microbial activity of both sheep and deer[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 202.9±8.0 °C at 760 mmHg |
| Melting Point | 35-40ºC |
| Molecular Formula | C10H18O |
| Molecular Weight | 154.249 |
| Flash Point | 73.9±0.0 °C |
| Exact Mass | 154.135757 |
| PSA | 20.23000 |
| LogP | 2.71 |
| Vapour Pressure | 0.1±0.9 mmHg at 25°C |
| Index of Refraction | 1.502 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 22-24/25-26 |
| RIDADR | UN 1325 4.1/PG 2 |
| WGK Germany | 3 |
| RTECS | DT5080000 |
|
Chocolate smells pink and stripy: Exploring olfactory-visual synesthesia.
Cogn Neurosci 6 , 77-88, (2015) Odors are often difficult to identify, and can be perceived either via the nose or mouth ("flavor"; not usually perceived as a "smell"). These features provide a unique opportunity to contrast concept... |
|
|
[GC-MS analysis and inhibitory activity of the essential oil extracted from the leaves of Lindera communis].
Zhong Yao Cai 22(3) , 128-31, (1999) The essential oil isolated from the dried leaves of Lindera communis was analyzed by means of gas chromatography-mass(GC-MS) technique, the structures of 23 chemical components were identified from it... |
|
|
Biosynthesis of monoterpenes. Enantioselectivity in the enzymatic cyclization of linalyl pyrophosphate to (-)-endo-fenchol.
J. Biol. Chem. 260(26) , 13901-8, (1985) The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of t... |
| 2-Fenchanol |
| 1,3,3-trimethyl-2-norbornanol |
| 1-Hydroxyfenchane |
| D-Fenchyl alcohol |
| fenchyl alcohol |
| FEMA 2480 |
| FENCHOL |
| Bicyclo[2.2.1]heptan-2-ol, 1,3,3-trimethyl- |
| 1,3,3-Trimethylbicyclo[2.2.1]heptan-2-ol |
