Rupatadine
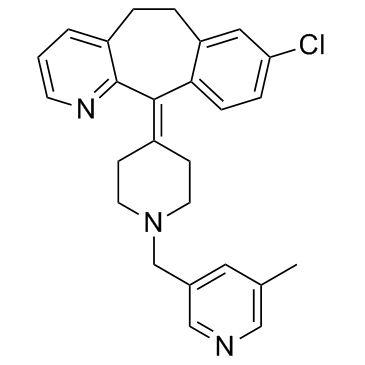
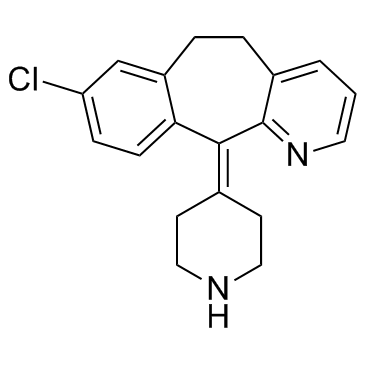
Rupatadine structure
|
Common Name | Rupatadine | ||
|---|---|---|---|---|
| CAS Number | 158876-82-5 | Molecular Weight | 415.958 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 586.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C26H26ClN3 | Melting Point | 58-61ºC | |
| MSDS | N/A | Flash Point | 308.4±30.1 °C | |
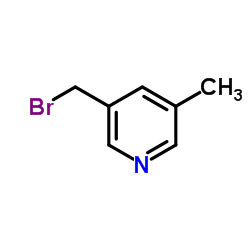
Use of RupatadineRupatadine (UR-12592) is a potent dual PAF/H1 antagonist with Ki of 0.55/0.1 uM(rabbit platelet membranes/guinea pig cerebellum membranes).IC50 value:Target: PAF/H1 antagonistin vitro: Rupatadine competitively inhibited histamine-induced guinea pig ileum contraction (pA2 = 9.29 +/- 0.06) without affecting contraction induced by ACh, serotonin or leukotriene D4 (LTD4). It also competitively inhibited PAF-induced platelet aggregation in washed rabbit platelets (WRP) (pA2 = 6.68 +/- 0.08) and in human platelet-rich plasma (HPRP) (IC50 = 0.68 microM), while not affecting ADP- or arachidonic acid-induced platelet aggregation [1]. The IC50 for rupatadine in A23187, concanavalin A and anti-IgE induced histamine release was 0.7+/-0.4 microM, 3.2+/-0.7 microM and 1.5+/-0.4 microM, respectively whereas for loratadine the IC50 was 2.1+/-0.9 microM, 4.0+/-1.3 M and 1.7+/-0.5 microM. SR-27417A exhibited no inhibitory effect [2].in vivo: Rupatadine blocked histamine- and PAF-induced effects in vivo, such as hypotension in rats (ID50 = 1.4 and 0.44 mg/kg i.v., respectively) and bronchoconstriction in guinea pigs (ID50 = 113 and 9.6 micrograms/kg i.v.). Moreover, it potently inhibited PAF-induced mortality in mice (ID50 = 0.31 and 3.0 mg/kg i.v. and p.o., respectively) and endotoxin-induced mortality in mice and rats (ID50 = 1.6 and 0.66 mg/kg i.v.) [1]. rupatadine treatment improved the declined lung function and significantly decreased animal death. Moreover, rupatadine was able not only to attenuate silica-induced silicosis but also to produce a superior therapeutic efficacy compared to pirfenidone, histamine H1 antagonist loratadine, or PAF antagonist CV-3988 [3]. |
| Name | 8-chloro-11-[1-[(5-methylpyridin-3-yl)methyl]piperidin-4-ylidene]-5,6-dihydrobenzo[1,2]cyclohepta[2,4-b]pyridine |
|---|---|
| Synonym | More Synonyms |
| Description | Rupatadine (UR-12592) is a potent dual PAF/H1 antagonist with Ki of 0.55/0.1 uM(rabbit platelet membranes/guinea pig cerebellum membranes).IC50 value:Target: PAF/H1 antagonistin vitro: Rupatadine competitively inhibited histamine-induced guinea pig ileum contraction (pA2 = 9.29 +/- 0.06) without affecting contraction induced by ACh, serotonin or leukotriene D4 (LTD4). It also competitively inhibited PAF-induced platelet aggregation in washed rabbit platelets (WRP) (pA2 = 6.68 +/- 0.08) and in human platelet-rich plasma (HPRP) (IC50 = 0.68 microM), while not affecting ADP- or arachidonic acid-induced platelet aggregation [1]. The IC50 for rupatadine in A23187, concanavalin A and anti-IgE induced histamine release was 0.7+/-0.4 microM, 3.2+/-0.7 microM and 1.5+/-0.4 microM, respectively whereas for loratadine the IC50 was 2.1+/-0.9 microM, 4.0+/-1.3 M and 1.7+/-0.5 microM. SR-27417A exhibited no inhibitory effect [2].in vivo: Rupatadine blocked histamine- and PAF-induced effects in vivo, such as hypotension in rats (ID50 = 1.4 and 0.44 mg/kg i.v., respectively) and bronchoconstriction in guinea pigs (ID50 = 113 and 9.6 micrograms/kg i.v.). Moreover, it potently inhibited PAF-induced mortality in mice (ID50 = 0.31 and 3.0 mg/kg i.v. and p.o., respectively) and endotoxin-induced mortality in mice and rats (ID50 = 1.6 and 0.66 mg/kg i.v.) [1]. rupatadine treatment improved the declined lung function and significantly decreased animal death. Moreover, rupatadine was able not only to attenuate silica-induced silicosis but also to produce a superior therapeutic efficacy compared to pirfenidone, histamine H1 antagonist loratadine, or PAF antagonist CV-3988 [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 586.4±50.0 °C at 760 mmHg |
| Melting Point | 58-61ºC |
| Molecular Formula | C26H26ClN3 |
| Molecular Weight | 415.958 |
| Flash Point | 308.4±30.1 °C |
| Exact Mass | 415.181519 |
| PSA | 29.02000 |
| LogP | 6.11 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.646 |
| Storage condition | 2-8℃ |
| HS Code | 2933990090 |
|---|
|
~% 
Rupatadine CAS#:158876-82-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 17 p. 2697 - 2703 |
|
~% 
Rupatadine CAS#:158876-82-5 |
| Literature: WO2006/103688 A1, ; Page/Page column 5-6 ; |
|
~% 
Rupatadine CAS#:158876-82-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 17 p. 2697 - 2703 |
|
~% 
Rupatadine CAS#:158876-82-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 17 p. 2697 - 2703 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Rupatadine [INN] |
| 5H-Benzo[5,6]cyclohepta[1,2-b]pyridine, 8-chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridinyl)methyl]-4-piperidinylidene]- |
| 8-Chloro-6,11-dihydro-11-(1-((5-methyl-3-pyridinyl)methyl)-4-piperidinylidene)-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| 8-Chloro-11-{1-[(5-methylpyridin-3-yl)methyl]piperidin-4-ylidene}-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| 8-chloro-6,11-dihydro-11-[1-[(5-methyl-3-pyridinyl)methyl]-4-piperidinylidene]-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| UR-12592 |
| 8-Chloro-11-{1-[(5-methyl-3-pyridinyl)methyl]-4-piperidinylidene}-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| 8-chloro-11-[1-[(5-methyl-3-pyridyl)methyl]piperidin-4-ylidene]-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| MFCD00926499 |
| 8-chloro-6,11-dihydro-11-(1-((5-methyl-3-pyridyl)methyl)-4-piperidylidene)-5h-benzo(5,6)cyclohepta(1,2-b)pyridine |
| 5H-Benzo(5,6)cyclohepta(1,2-b)pyridine, 8-chloro-6,11-dihydro-11-(1-((5-methyl-3-pyridinyl)methyl)-4-piperidinylidene)- |
| Rupatadinefumarate |
| 8-chloro-11-[1-[(5-methyl-3-pyridyl)methyl]-4-piperidyliden]-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| Rupatadine |
| UNII-2AE8M83G3E |
| 8-Chloro-11-(1-((5-methylpyridin-3-yl)methyl)piperidin-4-ylidene)-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine |
| Rupatadine Fumarate |