Methyl 4-formylbenzoate
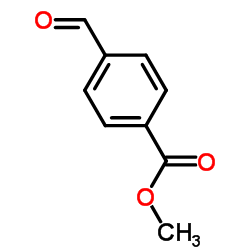
Methyl 4-formylbenzoate structure
|
Common Name | Methyl 4-formylbenzoate | ||
|---|---|---|---|---|
| CAS Number | 1571-08-0 | Molecular Weight | 164.158 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 265.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C9H8O3 | Melting Point | 59-63 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 122.5±22.7 °C | |
| Name | Methyl 4-Formylbenzoate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 265.0±0.0 °C at 760 mmHg |
| Melting Point | 59-63 °C(lit.) |
| Molecular Formula | C9H8O3 |
| Molecular Weight | 164.158 |
| Flash Point | 122.5±22.7 °C |
| Exact Mass | 164.047348 |
| PSA | 43.37000 |
| LogP | 2.05 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.558 |
| InChIKey | FEIOASZZURHTHB-UHFFFAOYSA-N |
| SMILES | COC(=O)c1ccc(C=O)cc1 |
| Stability | Stable, though possibly air-sensitive. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | insoluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| HS Code | 29183000 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2918300090 |
|---|---|
| Summary | 2918300090 other carboxylic acids with aldehyde or ketone function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Two main metabolites of gentiopicroside detected in rat plasma by LC-TOF-MS following 2,4-dinitrophenylhydrazine derivatization.
J. Pharm. Biomed. Anal. 107 , 1-6, (2015) The metabolism of gentiopicroside in vivo was studied by LC/MS following 2,4-dinitrophenylhydrazine derivatization for the first time. The ionization efficiency of the major metabolites erythrocentaur... |
|
|
New analytical method for the study of metabolism of swertiamarin in rats after oral administration by UPLC-TOF-MS following DNPH derivatization.
Biomed. Chromatogr. 29 , 1184-9, (2015) The metabolism of swertiamarin in vivo was studied by LC-MS following 2,4-dinitrophenylhydrazine derivatization. The ionization efficiency of the main metabolite erythrocentaurin was greatly enhanced ... |
|
|
Synthesis and discovery of high affinity folate receptor-specific glycinamide ribonucleotide formyltransferase inhibitors with antitumor activity.
J. Med. Chem. 51 , 5052, (2008) 6-Substituted classical pyrrolo[2,3-d]pyrimidine antifolates with a three- to six-carbon bridge between the heterocycle and the benzoyl-L-glutamate (compounds 2-5, respectively) were synthesized start... |
| METHYL PARA-FORMYLBENZOATE |
| p-Formylbenzoic acid methyl ester |
| p-Methoxycarbonylbenzaldehyde |
| Methyl Terephthalaldehydate |
| Methyl 4-formylbenzoate |
| methyl-4-formylbenzoate |
| 4-Formylbenzoic Acid Methyl Ester |
| Benzoic acid, 4-formyl-, methyl ester |
| Terephthalaldehydic Acid Methyl Ester |
| EINECS 216-385-5 |
| p-methoxycarbonyl benzaldehyde |
| MFCD00006950 |
 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:619-42-1
CAS#:619-42-1 CAS#:619-44-3
CAS#:619-44-3 CAS#:67-56-1
CAS#:67-56-1 CAS#:619-66-9
CAS#:619-66-9 CAS#:53148-13-3
CAS#:53148-13-3 CAS#:1679-64-7
CAS#:1679-64-7 CAS#:1774-35-2
CAS#:1774-35-2 CAS#:6908-41-4
CAS#:6908-41-4 CAS#:2417-72-3
CAS#:2417-72-3 CAS#:106918-32-5
CAS#:106918-32-5 CAS#:110137-64-9
CAS#:110137-64-9 CAS#:34040-64-7
CAS#:34040-64-7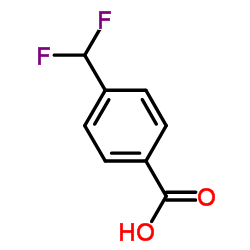 CAS#:55805-21-5
CAS#:55805-21-5 CAS#:4394-85-8
CAS#:4394-85-8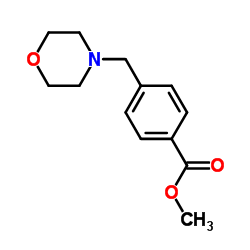 CAS#:68453-56-5
CAS#:68453-56-5 CAS#:42228-16-0
CAS#:42228-16-0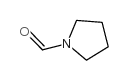 CAS#:3760-54-1
CAS#:3760-54-1
