ENT Efavirenz
Modify Date: 2025-09-18 18:05:35
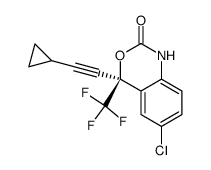
ENT Efavirenz structure
|
Common Name | ENT Efavirenz | ||
|---|---|---|---|---|
| CAS Number | 154801-74-8 | Molecular Weight | 315.67500 | |
| Density | 1.53g/cm3 | Boiling Point | 340.6ºC at 760 mmHg | |
| Molecular Formula | C14H9ClF3NO2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of ENT EfavirenzEfavirenz, (R)- is a nonnucleoside HIV-1 reverse transcriptase inhibitor. Efavirenz, (R)- is an antiviral. |
| Name | ent-Efavirenz |
|---|---|
| Synonym | More Synonyms |
| Density | 1.53g/cm3 |
|---|---|
| Boiling Point | 340.6ºC at 760 mmHg |
| Molecular Formula | C14H9ClF3NO2 |
| Molecular Weight | 315.67500 |
| Exact Mass | 315.02700 |
| PSA | 38.33000 |
| LogP | 4.21110 |
| Vapour Pressure | 8.53E-05mmHg at 25°C |
| Index of Refraction | 1.58 |
| 4-Chloro-2-methylsulfanyl-6-pyrrolidin-1-yl-pyrimidine-5-carbaldehyde |
| 4-chloro-2-methylthio-6-pyrrolidinylpyrimidine-5-carbaldehyde |
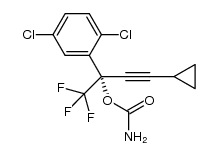 CAS#:1381993-90-3
CAS#:1381993-90-3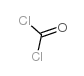 CAS#:75-44-5
CAS#:75-44-5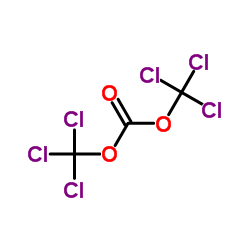 CAS#:32315-10-9
CAS#:32315-10-9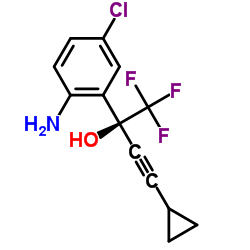 CAS#:209414-27-7
CAS#:209414-27-7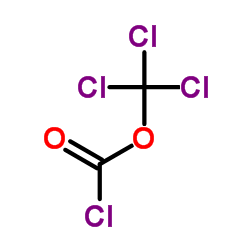 CAS#:503-38-8
CAS#:503-38-8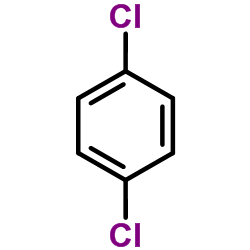 CAS#:106-46-7
CAS#:106-46-7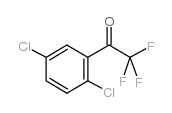 CAS#:886371-22-8
CAS#:886371-22-8 CAS#:6746-94-7
CAS#:6746-94-7