DL-5-Fluorotryptophan
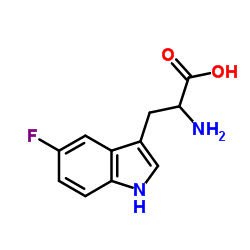
DL-5-Fluorotryptophan structure
|
Common Name | DL-5-Fluorotryptophan | ||
|---|---|---|---|---|
| CAS Number | 154-08-5 | Molecular Weight | 222.216 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 450.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C11H11FN2O2 | Melting Point | 265 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 226.4±28.7 °C | |
| Name | 5-fluorotryptophan |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 450.7±45.0 °C at 760 mmHg |
| Melting Point | 265 °C (dec.)(lit.) |
| Molecular Formula | C11H11FN2O2 |
| Molecular Weight | 222.216 |
| Flash Point | 226.4±28.7 °C |
| Exact Mass | 222.080460 |
| PSA | 79.11000 |
| LogP | 1.17 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.673 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | YN6825000 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin.
J. Med. Chem. 49(15) , 4616-22, (2006) Tryptophans at positions 4 and 7 of compstatin, a peptide complement inhibitor, are crucial for its interaction with C3. However, the nature of their involvement has not been studied to date. Here we ... |
|
|
Ionization potentials of fluoroindoles and the origin of nonexponential tryptophan fluorescence decay in proteins.
J. Am. Chem. Soc. 127(11) , 4104-13, (2005) This work reports an explanation for the unusual monoexponential fluorescence decay of 5-fluorotryptophan (5FTrp) in single-Trp mutant proteins [Broos, J.; Maddalena, F.; Hesp, B. H. J. Am. Chem. Soc.... |
|
|
In vivo synthesized proteins with monoexponential fluorescence decay kinetics.
J. Am. Chem. Soc. 126(1) , 22-3, (2004) Tryptophan, when in a protein, typically shows multiexponential fluorescence decay kinetics. Complex kinetics prevents a straightforward interpretation of time-resolved fluorescence protein data, part... |
| 2-amino-3-(5-fluoro-1H-indol-3-yl)propanoic acid |
| 5-Fluoro-DL-tryptophan |
| H-5-FLUORO-DL-TRP-OH |
| 5-Fluoro D,L-tryptophan |
| 5-fluoro-DL-Trp |
| 5-Fluoro-D,L-tryptophan |
| DL-5-FLUOROTRYPTOPHAN |
| MFCD00005649 |
| EINECS 205-822-5 |
| 5-Fluorotryptophan |
| 5-Fluoro-DL-tryptophane |
| Tryptophan, 5-fluoro- |
![2-acetamido-2-[(5-fluoro-1H-indol-3-yl)methyl]propanedioic acid Structure](https://image.chemsrc.com/caspic/173/363-37-1.png) CAS#:363-37-1
CAS#:363-37-1 CAS#:428-79-5
CAS#:428-79-5![acetylamino-[3-(4-fluoro-phenylhydrazono)-propyl]-malonic acid diethyl ester Structure](https://image.chemsrc.com/caspic/344/427-87-2.png) CAS#:427-87-2
CAS#:427-87-2 CAS#:576-16-9
CAS#:576-16-9