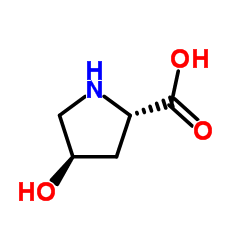
N-tert-Butoxycarbonyl-trans-4-hydroxy-D-proline

N-tert-Butoxycarbonyl-trans-4-hydroxy-D-proline structure
|
Common Name | N-tert-Butoxycarbonyl-trans-4-hydroxy-D-proline | ||
|---|---|---|---|---|
| CAS Number | 147266-92-0 | Molecular Weight | 231.246 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 390.9±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H17NO5 | Melting Point | 123ºC | |
| MSDS | Chinese USA | Flash Point | 190.2±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of N-tert-Butoxycarbonyl-trans-4-hydroxy-D-prolineN-tert-Butoxycarbonyl-trans-4-hydroxy-D-proline is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). N-tert-Butoxycarbonyl-trans-4-hydroxy-D-proline is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[1][2]. |
| Name | (2R,4S)-1-(tert-Butoxycarbonyl)-4-hydroxypyrrolidine-2-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | N-tert-Butoxycarbonyl-trans-4-hydroxy-D-proline is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). N-tert-Butoxycarbonyl-trans-4-hydroxy-D-proline is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PROTACs[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Non-cleavable |
| In Vitro | ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 390.9±42.0 °C at 760 mmHg |
| Melting Point | 123ºC |
| Molecular Formula | C10H17NO5 |
| Molecular Weight | 231.246 |
| Flash Point | 190.2±27.9 °C |
| Exact Mass | 231.110672 |
| PSA | 87.07000 |
| LogP | -0.71 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.531 |
| Storage condition | 2-8°C |
|
~88% 
N-tert-Butoxyca... CAS#:147266-92-0 |
| Literature: LG LIFE SCIENCES LTD. Patent: WO2010/56022 A2, 2010 ; Location in patent: Page/Page column 21 ; WO 2010/056022 A2 |
|
~99% 
N-tert-Butoxyca... CAS#:147266-92-0 |
| Literature: Fransson, Rebecca; McCracken, Alison N.; Chen, Bin; McMonigle, Ryan J.; Edinger, Aimee L.; Hanessian, Stephen ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 10 p. 969 - 973 |
|
~99% 
N-tert-Butoxyca... CAS#:147266-92-0 |
| Literature: Koskinen, Ari M. P.; Helaja, Juho; Kumpulainen, Esa T. T.; Koivisto, Jari; Mansikkamaeki, Heidi; Rissanen, Kari Journal of Organic Chemistry, 2005 , vol. 70, # 16 p. 6447 - 6453 |
|
~% 
N-tert-Butoxyca... CAS#:147266-92-0 |
| Literature: Journal of Organic Chemistry, , vol. 70, # 16 p. 6447 - 6453 |
|
~% 
N-tert-Butoxyca... CAS#:147266-92-0 |
| Literature: RSC Advances, , vol. 4, # 5 p. 2482 - 2490 |
|
~% 
N-tert-Butoxyca... CAS#:147266-92-0 |
| Literature: RSC Advances, , vol. 4, # 5 p. 2482 - 2490 |
|
~% 
N-tert-Butoxyca... CAS#:147266-92-0 |
| Literature: RSC Advances, , vol. 4, # 5 p. 2482 - 2490 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| (4S)-4-Hydroxy-1-{[(2-methyl-2-propanyl)oxy]carbonyl}-D-proline |
| Boc-trans-4-Hydroxy-D-proline |
| 1,2-Pyrrolidinedicarboxylic acid, 4-hydroxy-, 1-(1,1-dimethylethyl) ester, (2R,4S)- |
| N-Boc-trans-4-hydroxy-D-proline |
| MFCD01861341 |


![(1R,4R)-TERT-BUTYL 3-OXO-2-OXA-5-AZABICYCLO[2.2.1]HEPTANE-5-CARBOXYLATE structure](https://image.chemsrc.com/caspic/477/848488-70-0.png)

 CAS#:77450-03-4
CAS#:77450-03-4
