Vitisin A
Modify Date: 2025-08-26 19:12:05
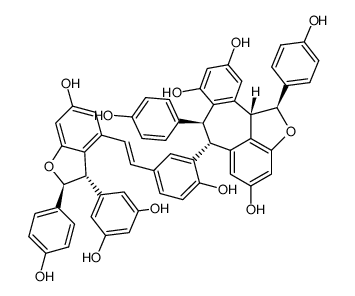
Vitisin A structure
|
Common Name | Vitisin A | ||
|---|---|---|---|---|
| CAS Number | 142449-89-6 | Molecular Weight | 906.93 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C56H42O12 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Vitisin AVitisin A has antioxidative, anticancer, antiapoptotic, neuroprotective and anti-inflammatory effects. Vitisin A inhibits LPS-induced NO and iNOS production via down-regulation of ERK1/2 and p38 and the NF-κB signal pathway. Vitisin A also inhibits adipocyte differentiation. Vitisin A is a resveratrol tetramer that can be isolated from Vitis vinifera roots[1][2][3]. |
| Name | (+)-vitisin A |
|---|---|
| Synonym | More Synonyms |
| Description | Vitisin A has antioxidative, anticancer, antiapoptotic, neuroprotective and anti-inflammatory effects. Vitisin A inhibits LPS-induced NO and iNOS production via down-regulation of ERK1/2 and p38 and the NF-κB signal pathway. Vitisin A also inhibits adipocyte differentiation. Vitisin A is a resveratrol tetramer that can be isolated from Vitis vinifera roots[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C56H42O12 |
|---|---|
| Molecular Weight | 906.93 |
| Exact Mass | 906.26800 |
| PSA | 220.76000 |
| LogP | 10.72160 |
| vitisin A |
| Vitisin A |
| (1S,6R,7S,11bS)-6-(5-{(E)-2-[(2S,3S)-3-(3,5-Dihydroxy-phenyl)-6-hydroxy-2-(4-hydroxy-phenyl)-2,3-dihydro-benzofuran-4-yl]-vinyl}-2-hydroxy-phenyl)-1,7-bis-(4-hydroxy-phenyl)-1,6,7,11b-tetrahydro-2-oxa-dibenzo[cd,h]azulene-4,8,10-triol |