N,N'-Bis(naphthalene-2-yl)-N,N'-bis(phenyl)benzidine
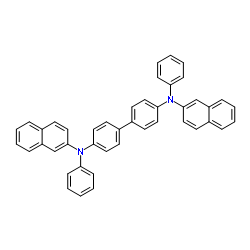
N,N'-Bis(naphthalene-2-yl)-N,N'-bis(phenyl)benzidine structure
|
Common Name | N,N'-Bis(naphthalene-2-yl)-N,N'-bis(phenyl)benzidine | ||
|---|---|---|---|---|
| CAS Number | 139255-17-7 | Molecular Weight | 588.738 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 772.5±53.0 °C at 760 mmHg | |
| Molecular Formula | C44H32N2 | Melting Point | 174-176ºC | |
| MSDS | USA | Flash Point | 340.8±18.8 °C | |
| Name | N-[4-[4-(N-naphthalen-2-ylanilino)phenyl]phenyl]-N-phenylnaphthalen-2-amine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 772.5±53.0 °C at 760 mmHg |
| Melting Point | 174-176ºC |
| Molecular Formula | C44H32N2 |
| Molecular Weight | 588.738 |
| Flash Point | 340.8±18.8 °C |
| Exact Mass | 588.256531 |
| PSA | 6.48000 |
| LogP | 13.48 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.733 |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~88% 
N,N'-Bis(naphth... CAS#:139255-17-7 |
| Literature: Liu, Yu-Hua; Chen, Chen; Yang, Lian-Ming Tetrahedron Letters, 2006 , vol. 47, # 52 p. 9275 - 9278 |
|
~83% 
N,N'-Bis(naphth... CAS#:139255-17-7 |
| Literature: Maiti; Wang; Cheng; Huang; Chao Journal of the Chinese Chemical Society, 2001 , vol. 48, # 6 A p. 1059 - 1064 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Clean synthesis of triarylamines: Buchwald-Hartwig reaction in water with amphiphilic resin-supported palladium complexes.
Chem. Commun. (Camb.) 46 , 1103-1105, (2010) Catalytic aromatic amination was achieved in water under heterogeneous conditions by the use of palladium complexes anchored to the amphiphilic PS-PEG resin with little palladium leaching to provide a... |
|
|
Synthesis and electroluminescence of blue fluorescent diarylaminofluorene derivatives for organic light-emitting diodes.
J. Nanosci. Nanotechnol. 9 , 7056-7060, (2009) A series of seven diarylaminofluorene-derived fluorescent molecules demonstrating a blue emission in organic light-emitting diodes (OLEDs) were synthesized via the Horner-Wadsworth-Emmons reaction and... |
|
|
Interfacial Charge Transfer and Charge Generation in Organic Electronic Devices Matsushima T, et al.
Org. Electron. 12(3) , 520-528, (2011)
|
| [1,1'-Biphenyl]-4,4'-diamine, N,N-di-2-naphthalenyl-N,N-diphenyl- |
| N4,N4'-Di(naphthalen-2-yl)-N4,N4'-diphenyl-[1,1'-biphenyl]-4,4'-diamine |
| N,N'-di(naphthalene-2-yl)-N,N'-diphenyl-benzidine |
| N,N'-Di(2-naphthyl-N,N'-diphenyl)-1,1'-biphenyl-4,4'-diamine |
| N,N'-Di(2-naphthyl)-N,N'-diphenylbiphenyl-4,4'-diamine |
| 4,4'-bis[N-(2-naphthyl)-N-phenylamino]biphenyl |
| N,N'-Bis(naphthalene-2-yl)-N,N'-bis(phenyl)benzidine |
| N,N'-bis(naphthalen-2-yl)-N,N'-bis(phenyl)benzidine |
| N,N'-dinaphthyl-N,N'-diphenyl-1,1'-biphenyl-4,4'-diamine |
| N,N'-diphenyl-N,N'-bis(2-naphthyl)-4,4'-diaminobiphenyl |
| N,N'-Di(2-naphthyl)-N,N'-diphenyl-4,4'-biphenyldiamine |
| N,N-Bis(Naphthalene-2-yl)-N,N-Bis(Phenyl)Benzidine |