Tripterifordin
Modify Date: 2024-01-08 18:10:43
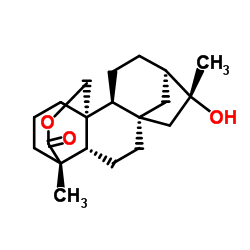
Tripterifordin structure
|
Common Name | Tripterifordin | ||
|---|---|---|---|---|
| CAS Number | 139122-81-9 | Molecular Weight | 318.450 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 485.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C20H30O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 198.6±21.5 °C | |
Use of TripterifordinTripterifordin, isolated from the roots of Tripterygium wilfordii, possesses significant anti-HIV replication activities in H9 lymphocyte cells with an EC50 value of 3100 nM, respectively[1]. |
| Name | tripterifordin |
|---|---|
| Synonym | More Synonyms |
| Description | Tripterifordin, isolated from the roots of Tripterygium wilfordii, possesses significant anti-HIV replication activities in H9 lymphocyte cells with an EC50 value of 3100 nM, respectively[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 485.1±45.0 °C at 760 mmHg |
| Molecular Formula | C20H30O3 |
| Molecular Weight | 318.450 |
| Flash Point | 198.6±21.5 °C |
| Exact Mass | 318.219482 |
| PSA | 46.53000 |
| LogP | 3.20 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.570 |
| InChIKey | KLMZPLYXGZZBCX-XGRAKDAMSA-N |
| SMILES | CC1(O)CC23CCC4C5(C)CCCC4(COC5=O)C2CCC1C3 |
| Hazard Codes | Xi |
|---|
| (8α,10α,13α,16β)-16-Hydroxy-18,20-epoxykauran-18-one |
| (5β,8α,10α,13α,16β)-16-Hydroxy-18,20-epoxykauran-18-one |
| Tripterifordin |