| In Vitro |
FL118 (0-200 nM; 24, 48 and 72 h ) inhibits the cell proliferation of ES-2 and SK-O-V3 cells[1]. FL118 (0-100 nM; 0 and 24 h) inhibits the migration of ES-2 and SK-O-V3 cells[1]. FL118 (0-100 nM; 48 h) affects the expression level of cytoglobin (CYGB)[1]. FL118 (10 and 100 nM; 48 h) inhibits PI3K/AKT/mTOR signaling pathway, and affects the expression level of vimentin and E-cadherin in ovarian cancer cells[1]. FL118 (0-100 nM; 6 and 24 h) can dephosphorylate and degrade DDX5[2]. FL118 (0-500 nM; 24, 48, 72 h) regulates survivin, McL-1, XIAP, cIAP2, c-MYc and mKras by regulating DDX5[2]. FL118 (0-1 μM, 24 h) shows significant cytotoxic activity against the three tumor cell lines (A549, MDA-MB-231, and RM-1 cells)[3]. FL118 (0-10 nM, 48 h) increases the production of PARP cleavage, and induces apoptosis in A549[3]. FL118 (0-10 nM, 48 h) arrests A549 cells mainly at the G2/M phase[3]. Western Blot Analysis[1] Cell Line: ES-2 and SK-O-V3 cell lines Concentration: 10 and 100 nM Incubation Time: 48 h Result: Effectively inhibited the activation of PI3K/AKT/mTOR signaling pathway in ovarian cancer cells and also inhibited the migration of ES-2 and SK-O-V3 cells. Cell Migration Assay [1] Cell Line: ES-2 and SK-O-V3 cell lines Concentration: 0, 10 and 100 nM Incubation Time: 0 and 24 h Result: Inhibited the migration of ES-2 and SK-O-V3 cells dose-dependenly. RT-PCR[1] Cell Line: ES-2 and SK-O-V3 cell lines Concentration: 0, 10 and 100 nM Incubation Time: 48 h Result: Promoted CYGB expression. Cell Proliferation Assay[1] Cell Line: ES-2 and SK-O-V3 cell lines Concentration: 0, 1, 10, 50, 100 and 200 nM Incubation Time: 24, 48 and 72 h Result: Inhibited the cell proliferation of ES-2 and SK-O-V3 cells time- and dose-dependently. Western Blot Analysis[2] Cell Line: SW620 and Mia Paca-2 Concentration: 0, 10 and 100 nM Incubation Time: 6 and 24 h Result: Induced dephosphorylation of DDX5 through the ubiquitin-proteasome degradation pathway and degraded DDX5 time-dependently. Western Blot Analysis[2] Cell Line: PDAC Panc1, CRC HCT-8, SW620, Mia Paca-2, Panc-1, HCT-8 cell lines Concentration: 0, 10, 100 and 500 nM Incubation Time: 24, 48, 72 h Result: Controled the expression of survivin, Mcl-1, XIAP, cIAP2, c-Myc and mKras by regulated DDX5, as an upstream master regulator in cancer development and malignant networks. Cell Cytotoxicity Assay[3] Cell Line: A549, MDA-MB-231, RM-1 Concentration: 0-1 μM Incubation Time: 24 h Result: Showed cytotoxicity in A-549 (human lung carcinoma), MDA-MB-231 (human breast carcinoma) and RM-1 (mouse prostate carcinoma), with IC50 values of 8.94 ± 1.54 , 24.73 ± 13.82, and 69.19 ± 8.34 nM, respectively. Apoptosis Analysis[3] Cell Line: A549 cells Concentration: 0, 2.5, 5, 10 nM Incubation Time: 48 h Result: Resulted in the downregulation of survivin. Increased the production of PARP cleavage in a concentration-dependent manner, which is the hallmark of apoptosis. Induced apoptosis in A549. Cell Cycle Analysis[3] Cell Line: A549 cells Concentration: 0, 2.5, 5, 10 nM Incubation Time: 48 h Result: Increased G2/M cell population in a concentration-dependent manner, and arrested A549 cells mainly at the G2/M phase.
|
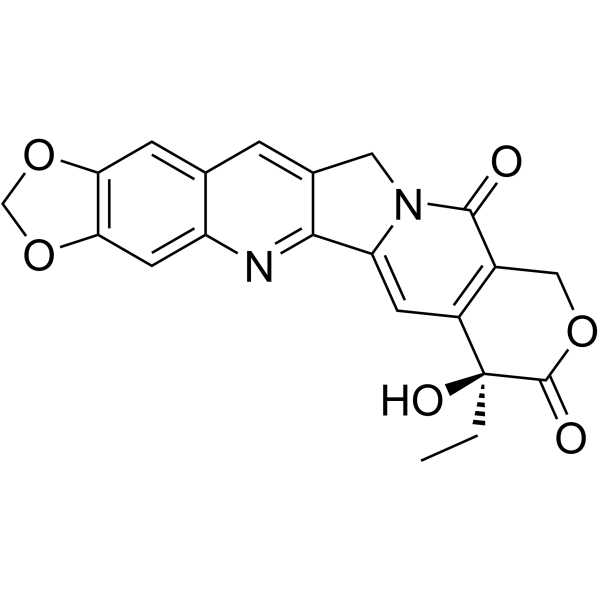
![(S)-4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione structure](https://www.chemsrc.com/caspic/225/110351-94-5.png)


![(S)-4-ethyl-4-hydroxy-6-iodo-3-oxo-1H-pyrano[3,4-c]-8-pyridone structure](https://www.chemsrc.com/caspic/163/173442-34-7.png)
![(S)-4-Ethyl-4-hydroxy-6-iodo-3-oxo-7-propargyl-1H-pyrano[3,4-c]-8-pyridone structure](https://www.chemsrc.com/caspic/174/174092-80-9.png)
![(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one structure](https://www.chemsrc.com/caspic/029/174092-79-6.png)
![4-(trimethylsilyl)benzo[d][1,3]dioxole-5-carbaldehyde structure](https://www.chemsrc.com/caspic/454/181780-65-4.png)
![(+/-)-4-ethyl-4-hydroxy-8-methoxy-6-(trimethylsilyl)-1,4-dihydro-3H-pyrano[3,4-c]pyridin-3-one structure](https://www.chemsrc.com/caspic/404/869097-09-6.png)