famoxadone
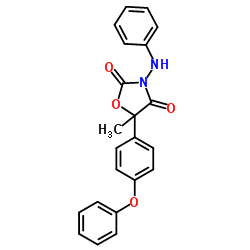
famoxadone structure
|
Common Name | famoxadone | ||
|---|---|---|---|---|
| CAS Number | 131807-57-3 | Molecular Weight | 374.389 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 491.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C22H18N2O4 | Melting Point | 140.3-141.8ºC | |
| MSDS | N/A | Flash Point | 250.9±31.5 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of famoxadoneFamoxadone (DPX-JE874) is a fungicide acting against a broad spectrum of fungi and is widely used in Integrated Pest Management strategies in different agricultural crops[1]. |
| Name | famoxadone |
|---|---|
| Synonym | More Synonyms |
| Description | Famoxadone (DPX-JE874) is a fungicide acting against a broad spectrum of fungi and is widely used in Integrated Pest Management strategies in different agricultural crops[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 491.3±55.0 °C at 760 mmHg |
| Melting Point | 140.3-141.8ºC |
| Molecular Formula | C22H18N2O4 |
| Molecular Weight | 374.389 |
| Flash Point | 250.9±31.5 °C |
| Exact Mass | 374.126648 |
| PSA | 67.87000 |
| LogP | 4.76 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.659 |
| InChIKey | PCCSBWNGDMYFCW-UHFFFAOYSA-N |
| SMILES | CC1(c2ccc(Oc3ccccc3)cc2)OC(=O)N(Nc2ccccc2)C1=O |
| Storage condition | 0-6°C |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302-H312-H319-H332 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn,N,F |
| Risk Phrases | R48/22 |
| Safety Phrases | S46 |
| RIDADR | UN1648 3/PG 2 |
| Hazard Class | 9.0 |
|
~% 
famoxadone CAS#:131807-57-3 |
| Literature: US5948805 A1, ; |
|
~% 
famoxadone CAS#:131807-57-3 |
| Literature: Pest Management Science, , vol. 57, # 2 p. 143 - 152 |
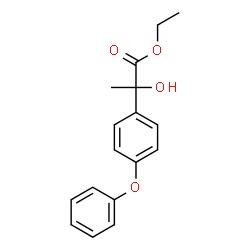
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Development and validation of one-step ultrasound-assisted extraction for simultaneous determination of multiclass fungicides in soils.
J. AOAC Int. 98(1) , 192-200, (2015) A rapid, efficient, and simple one-step ultrasound-assisted extraction (UAE) method was developed for the analysis of seven fungicides (cymoxanil, metalaxyl, mandipropamid, folpet, chlorothalonil, kre... |
|
|
Triplet state of the semiquinone-Rieske cluster as an intermediate of electronic bifurcation catalyzed by cytochrome bc1.
Biochemistry 52 , 6388-95, (2013) Efficient energy conversion often requires stabilization of one-electron intermediates within catalytic sites of redox enzymes. While quinol oxidoreductases are known to stabilize semiquinones, one of... |
| 5-Methyl-5-(4-phenoxyphenyl)-3-(phenylamino)-1,3-oxazolidine-2,4-dione |
| (RS)-3-anilino-5-methyl-5-(4-phenoxyphenyl)-1,3-oxazolidine-2,4-dione |
| 3-Anilino-5-methyl-5-(4-phenoxyphenyl)oxazolidine-2,4-dione |
| MFCD03427409 |
| rac-(5R)-3-anilino-5-methyl-5-(4-phenoxyphenyl)-1,3-oxazolidine-2,4-dione |
| T5OVNV EHJ CMR& E1 ER DOR |
| Famoxate |
| 2,4-Oxazolidinedione, 5-methyl-5-(4-phenoxyphenyl)-3-(phenylamino)- |
| DPX-JE 874 |
| famoxadone |
| 3-Anilino-5-methyl-5-(4-phenoxyphenyl)-1,3-oxazolidine-2,4-dione |
| 5-methyl-5-(4-phenoxyphenyl)-3-(phenylamino)-2,4-oxazolidinedione |
| EINECS 200-835-2 |
| Famoxadon |