Concizumab
Modify Date: 2025-08-26 19:20:00
Concizumab structure
|
Common Name | Concizumab | ||
|---|---|---|---|---|
| CAS Number | 1312299-39-0 | Molecular Weight | N/A | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | N/A | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
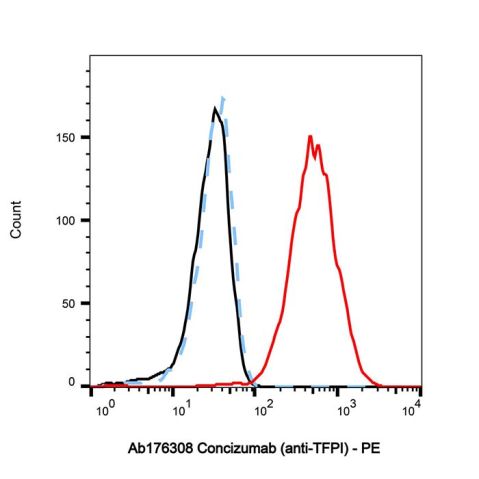
Use of ConcizumabConcizumab is an anti-TFPI monoclonal antibody (IgG4 type) that binds to the Kunitz-type protease inhibitor (KPI) 2 structural domain of TFPI, thereby blocking the interaction of this structural domain with the FXa active site. Concizumab can be used in the study of haemophilia[1][2]. |
| Name | Concizumab |
|---|
| Description | Concizumab is an anti-TFPI monoclonal antibody (IgG4 type) that binds to the Kunitz-type protease inhibitor (KPI) 2 structural domain of TFPI, thereby blocking the interaction of this structural domain with the FXa active site. Concizumab can be used in the study of haemophilia[1][2]. |
|---|---|
| Related Catalog | |
| Target |
TFPI[1][2]. |
| In Vitro | Concizumab 以浓度依赖性方式增加凝血酶峰值[1]。 |
| In Vivo | Concizumab (i.v./s.c.) 阻断 TFPI 与食蟹猴中 FXa 活性位点的相互作用[2]。 Animal Model: Cynomolgus monkeys (target mediated drug disposition (TMDD) model)[2]. Dosage: 20mg/kg or 200 mg/kg Administration: Intravenous injection/subcutaneous injection Result: Showed a high bioavailability (93%) and the absorption half-life is estimated to be 72 h. |
| References |
| No Any Chemical & Physical Properties |