Romidepsin (FK228, Depsipeptide)
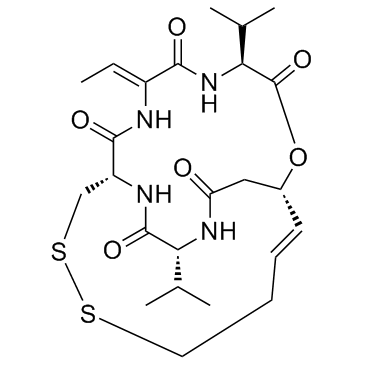
Romidepsin (FK228, Depsipeptide) structure
|
Common Name | Romidepsin (FK228, Depsipeptide) | ||
|---|---|---|---|---|
| CAS Number | 128517-07-7 | Molecular Weight | 540.696 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 942.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C24H36N4O6S2 | Melting Point | 219-224°C | |
| MSDS | USA | Flash Point | 524.0±34.3 °C | |
Use of Romidepsin (FK228, Depsipeptide)Romidepsin is a potent HDAC1 and HDAC2 inhibitor with IC50s of 36 and 47 nM, respectively. |
| Name | romidepsin |
|---|---|
| Synonym | More Synonyms |
| Description | Romidepsin is a potent HDAC1 and HDAC2 inhibitor with IC50s of 36 and 47 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
HDAC1:36 nM (IC50) HDAC2:47 nM (IC50) HDAC4:510 nM (IC50) HDAC6:14000 nM (IC50) |
| In Vitro | Romidepsin potently inhibits HDAC1 and HDAC2 (IC50=36, 47 nM, respectively). Romidepsin has slightly inhibitory effects against HDAC4 and HDAC6 (IC50=510, 14000 nM, respectively). Romidepsin induces histone acetylation and p21 expression with an EC50 of 3.0 nM. Romidepsin is more strongly than redFK with EC50 of 11 nM due to the instability of redFK in HeLa cells[1]. Romidepsin is 100 times more potent than TSA and 1,000,000 times more potent than butyrate in inhibiting the proliferation of the A549 cells. Romidepsin causes mitotic arrest, and that the treatment with HDIs causes defects in chromosome segregation in mitosis[2]. Romidepsin inhibits the growth of U-937, K562, and CCRF-CEM cells with IC50 values of 5.92 nM, 8.36 nM, and 6.95 nM, respectively[3]. |
| In Vivo | In a scid mouse lymphoma model, romidepsin treated mice once or twice a week survive longer than control mice, with median survival times of 30.5 (0.56 mg/kg) and 33 days (0.32 mg/kg), respectively (vs. 20 days in control mice). Remarkably, 2 out of 12 mice treated with romidepsin (0.56 mg/kg once or twice a week) survive past the observation period of 60 days. The apoptotic population of U-937 cells increases to 37.7% after 48 hr of treatment with romidepsin in a time dependent manner. In addition, romidepsin induces G1 and G2/M arrest and the differentiation of U-937 cells to the CD11b(+)/CD14(+) phenotype. Expression of p21(WAF1/Cip1) and gelsolin mRNA increases up to 654- and 152-fold, respectively, after 24 hr of treatment with romidepsin. Romidepsin causes histone acetylation in p21(WAF1/Cip1) promoter regions, including the Sp1-binding sites[3]. |
| Kinase Assay | For the enzyme assay, 10 μL of [3H]acetyl-labeled histones (25,000 cpm/10 μg) are added to 90 μL of the HDAC enzyme fraction extracted from 293T cells overexpressing HDAC1 or HDAC2 in the presence of increasing concentrations of Romidepsin, and the mixture is incubated at 37°C for 15 minutes. The enzyme reaction is linear for at least 1 hour. The reaction is stopped by the addition of 10 μL of concentrated HCl. The released [3H]acetic acid is extracted with 1 mL of ethylacetate, and 0.9 mL of the solvent layer is taken into 5 mL of aqueous counting scintillant II solution for determination of radioactivity. The IC50 values are determined from at least three independent dose-response curves. |
| Cell Assay | Cells are exposed to various concentrations of Romidepsin for 72 hours in 96-well plates. 20 μL of 5 mg/mL MTT solution in PBS is added to each well for 4 hours. After removal of the medium, 170 μL of DMSO is added to each well to dissolve the formazan crystals. The absorbance at 540 nm is determined. In addition, cells are incubated with trypan blue, and the numbers of blue (dead) cells and transparent (live) cells are counted in a hemocytometer. For cell cycle analysis, cells are incubated for 30 minutes in propidium iodide staining solution containing 0.05 mg/mL propidium iodide, 1 mM EDTA, 0.1% Triton X-100, and 1 mg/mL RNase A in PBS. The suspension is then passed through a nylon mesh filter and analyzed on a Becton Dickinson FACScan. |
| Animal Admin | Briefly, male scidmice are injected i.p. with 150 mg/kg of cyclophosphamide. All the mice injected with cyclophosphamide alone survive over the observation period. Twenty-four hours after cyclophosphamide injection, mice are inoculated i.p. with 1×107 U-937 cells in 500 μL PBS. Twenty-four hours after tumor cell inoculation, the mice are treated i.p. with or without FK228 at doses ranging from 0.1 to 1 mg/kg, once or twice a week. Control mice are treated with 10% HCO60 saline twice a week. Six and twelve mice are used for FK228-treated and control groups, respectively. The observation period is 60 days after cell inoculation, and the antitumor effect is evaluated by survival time, which is analyzed for statistical significance by Peto’s test. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 942.8±65.0 °C at 760 mmHg |
| Melting Point | 219-224°C |
| Molecular Formula | C24H36N4O6S2 |
| Molecular Weight | 540.696 |
| Flash Point | 524.0±34.3 °C |
| Exact Mass | 540.207642 |
| PSA | 193.30000 |
| LogP | 0.95 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.529 |
| Storage condition | Amber Vial, -20°C Freezer, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | YV9399000 |
| HS Code | 29349990 |
|
Induction of MAPK- and ROS-dependent autophagy and apoptosis in gastric carcinoma by combination of romidepsin and bortezomib.
Oncotarget 7 , 4454-67, (2016) Proteasome inhibitors and histone deacetylase (HDAC) inhibitors can synergistically induce apoptotic cell death in certain cancer cell types but their combinatorial effect on the induction of autophag... |
|
|
Non-epigenetic function of HDAC8 in regulating breast cancer stem cells by maintaining Notch1 protein stability.
Oncotarget 7 , 1796-807, (2016) Here, we report a novel non-epigenetic function of histone deacetylase (HDAC) 8 in activating cancer stem cell (CSC)-like properties in breast cancer cells by enhancing the stability of Notch1 protein... |
|
|
Valproic acid potentiates the anticancer activity of capecitabine in vitro and in vivo in breast cancer models via induction of thymidine phosphorylase expression.
Oncotarget 7 , 7715-31, (2016) The prognosis of patients with metastatic breast cancer remains poor, and thus novel therapeutic approaches are needed. Capecitabine, which is commonly used for metastatic breast cancer in different s... |
| (1S,4S,7Z,10S,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone |
| 2-Oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone, 7-ethylidene-4,21-bis(1-methylethyl)-, (1S,4S,7Z,10S,21R)- |
| 2-Oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone, 7-ethylidene-4,21-bis(1-methylethyl)-, (1S,4S,7Z,10S,16Z,21R)- |
| Chromadax |
| Depsipeptide |
| (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone |
| Romidepsin (FK228,depsipeptide) |
| FK-228 |
| (1S,4S,7Z,10S,16Z,21R)-7-Ethylidene-4,21-diisopropyl-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone |
| Romidepsin |
| (1S,4S,7Z,10S,21R)-7-Ethylidene-4,21-diisopropyl-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone |
| Istodax |