rac Fosfomycin-13C3 Benzylamine Salt
Modify Date: 2025-10-08 11:29:52
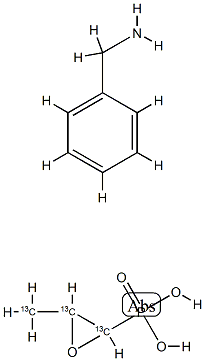
rac Fosfomycin-13C3 Benzylamine Salt structure
|
Common Name | rac Fosfomycin-13C3 Benzylamine Salt | ||
|---|---|---|---|---|
| CAS Number | 1216461-18-5 | Molecular Weight | 248.239101 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C10H16NO4P | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of rac Fosfomycin-13C3 Benzylamine Salt(Rac)-Fosfomycin (benzylamine)-13C3 is the 13C labeled Fosfomycin[1]. Fosfomycin (MK-0955) is a broad-spectrum antibiotic. Fosfomycin can cross blood-brain barrier penetrating, and irreversibly inhibits an early stage in cell wall synthesis. Fosfomycin shows anti-bacteria activity for a range of bacteria, including multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) bacteria[2][3]. |
| Name | yfochwjdruxoiq-burviegvsa-n |
|---|
| Description | (Rac)-Fosfomycin (benzylamine)-13C3 is the 13C labeled Fosfomycin[1]. Fosfomycin (MK-0955) is a broad-spectrum antibiotic. Fosfomycin can cross blood-brain barrier penetrating, and irreversibly inhibits an early stage in cell wall synthesis. Fosfomycin shows anti-bacteria activity for a range of bacteria, including multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) bacteria[2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
[2]. Falagas ME, et al. Fosfomycin. Clin Microbiol Rev. 2016 Apr. 29(2):321-47. |
| Molecular Formula | C10H16NO4P |
|---|---|
| Molecular Weight | 248.239101 |
| InChIKey | YFOCHWJDRUXOIQ-BURVIEGVSA-N |
| SMILES | CC1OC1P(=O)(O)O.NCc1ccccc1 |