Valspodar
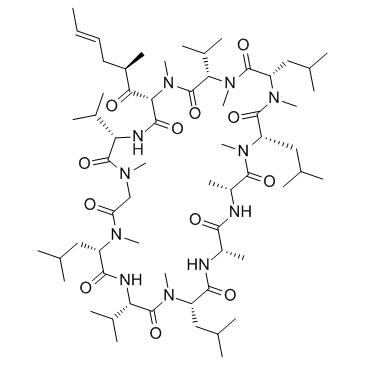
Valspodar structure
|
Common Name | Valspodar | ||
|---|---|---|---|---|
| CAS Number | 121584-18-7 | Molecular Weight | 1214.622 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 1290.1±65.0 °C at 760 mmHg | |
| Molecular Formula | C63H111N11O12 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 734.0±34.3 °C | |
Use of ValspodarValspodar is a P-glycoprotein (P-gp) inhibitor widely used as overcoming multidrug resistance modulator. |
| Name | Valspodar |
|---|---|
| Synonym | More Synonyms |
| Description | Valspodar is a P-glycoprotein (P-gp) inhibitor widely used as overcoming multidrug resistance modulator. |
|---|---|
| Related Catalog | |
| In Vitro | Valspodar (PSC 833) has no cytotoxicity effects at up to the concentration of 0.75 μg/mL. Valspodar (0.25, 0.5 and 0.75 μg/mL) and DOX-L are added to the DOX resistant cells, and cell kill efficacy of MDR cell type increases significantly when valspodar is administered alongside DOX-L. Valspodar (0.5 and 0.75 μg/mL), in combination with all concentrations of DOX, are most toxic and kill more than 70% of the resistant cells[1]. Pretreatment with PSC833 decreases the IC50 value of mitoxantrone in MDA-MB-435mdr cells to 0.4±0.02 μM in MDR cells and almost completely reverses the resistance of MDR cells to mitoxantrone[3]. |
| In Vivo | valspodar (10 mg/kg, o.p.) exhibits minimal blood-cell partitioning as reflected in its low mean blood-to-plasma ratio of approximately 0.52. Valspodar displays properties of slow clearance and a large volume of distribution. Valspodar shows properties of low hepatic extraction and wide distribution, similar to that of its structural analogue cyclosporine A[2]. Preadministration of PSC833 to mice increases mitoxantrone fluorescent intensity in MDR tumor to 94% of that in the wild-type tumors[3]. |
| Cell Assay | The in vitro cytotoxicity of various formulations against T47D/TAMR-6 cells is investigated by MTT assay. A 104 T47D/TAMR-6 cells are cultured in 96-well plate containing RPMI medium and incubated overnight to allow cell attachment. After 48 hours incubation, fresh medium containing serial concentration of various drug formulations, including free DOX, DOX-L, mixture of DOX-L and free Valspodar (PSC 833), mixture of DOX-L and PSC-L and DOX/PSC-L are added. The plates are then incubated for an additional 48 hours before washing with normal saline followed by adding MTT solution (0.5 mg/mL) to each well, and incubated for 4 h at 37°C. Then, the medium is removed, and DMSO is added to dissolve the formazan crystals. The plates are mildly shaken for 10 min to ensure the dissolution of formazan. The formazan dye is measured spectrophotometrically using microplate reader at 570 nm with reference standard of 690 nm as described before. |
| Animal Admin | Male Sprague–Dawley rats (250-350 g) are housed in temperature-controlled rooms with 12 h of light per day. The animals had free access to food and water prior to experimentation. Rats are divided into two groups: one group (n=6) receives intravenous dose (5 mg/kg) of valspodar and the other group administered valspodar orally (10 mg/kg). Stereoselective pharmacokinetics of desbutylhalofantrine, a metabolite of halofantrine, in the rat after administration of the racemic metabolite or parent drug. After surgery, the rats are transferred to their regular holding cages and allowed free access to water, but food is withheld overnight. The next morning, rats are transferred to the metabolic cages and dosed with valspodar. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 1290.1±65.0 °C at 760 mmHg |
| Molecular Formula | C63H111N11O12 |
| Molecular Weight | 1214.622 |
| Flash Point | 734.0±34.3 °C |
| Exact Mass | 1213.841309 |
| PSA | 275.64000 |
| LogP | 4.10 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.467 |
| InChIKey | YJDYDFNKCBANTM-QCWCSKBGSA-N |
| SMILES | CC=CCC(C)C(=O)C1C(=O)NC(C(C)C)C(=O)N(C)CC(=O)N(C)C(CC(C)C)C(=O)NC(C(C)C)C(=O)N(C)C(CC(C)C)C(=O)NC(C)C(=O)NC(C)C(=O)N(C)C(CC(C)C)C(=O)N(C)C(CC(C)C)C(=O)N(C)C(C(C)C)C(=O)N1C |
| Storage condition | 2-8℃ |
| RIDADR | NONH for all modes of transport |
|---|
|
Potential role of acid ceramidase in conversion of cytostatic to cytotoxic end-point in pancreatic cancer cells.
Cancer Chemother. Pharmacol. 71(3) , 635-45, (2013) Acid ceramidase (AC) occupies an important place in the control of cancer cell proliferation. We tested the influence of AC inhibition on the effects of PSC 833, a P-glycoprotein antagonist with poten... |
|
|
A tamoxifen derivative, N,N-diethyl-2-[4-(phenylmethyl) phenoxy] ethanamine, selectively targets P-glycoprotein-positive multidrug resistant Chinese hamster cells.
Biochem. Pharmacol. 90(2) , 107-14, (2014) DPPE, a tamoxifen derivative with antihistamine activity, was previously shown to potentiate the toxicity of chemotherapeutic drugs. Recently, a Phase III clinical study using doxorubicin with DPPE de... |
|
|
Rapid detection of ABC transporter interaction: potential utility in pharmacology.
J. Pharmacol. Toxicol. Methods 63(3) , 217-22, (2011) The ATP-binding cassette (ABC) transporters P-glycoprotein (P-gp/ABCB1), multidrug resistance-associated protein 1 (MRP1/ABCC1), and breast cancer resistance protein (BCRP/ABCG2) are known to transpor... |
| (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-6,9,18,24-Tetraisobutyl-3,21,30-triisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-33-[(2R,4E)-2-methyl-4-hexenoyl]-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone |
| Sdz-nvi-085 |
| Specific MDR1 P-gp Inhibitor |
| 6-((R-(E))-6,7-Didehydro-N,4-dimethyl-3-oxo-L-2-aminooctanoic acid)-7-L-valine-cyclosporin A |
| (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-6,9,18,24-Tetraisobutyl-3,21,30-triisopropyl-1,4,7,10,12,15,19,25,28-nonamethyl-33-[(2R,4E)-2-methylhex-4-enoyl]-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone |
| (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-1,4,7,10,12,15,19,25,28-nonamethyl-33-[(2R,4E)-2-methylhex-4-enoyl]-6,9,18,24-tetrakis(2-methylpropyl)-3,21,30-tri(propan-2-yl)-1,4,7,10,13,16,19,22,25,28,31-undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone |
| 1,4,7,10,13,16,19,22,25,28,31-Undecaazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone, 1,4,7,10,12,15,19,25,28-nonamethyl-3,21,30-tris(1-methylethyl)-33-[(2R,4E)-2-methyl-1-oxo-4-hexen-1-yl]-6,9,18,24-tetrakis(2-methylpropyl)-, (3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)- |
| PSC833 |
| Cyclo[[(2S,4R,6E)-4-Methyl-2-(methylamino)-3-oxo-6-octenoyl]-L-valyl-N-methylglucyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-N-methyl-L-valyl] |
| Valspodar |