Dabrafenib Mesylate
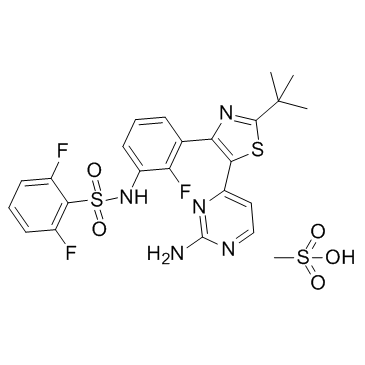
Dabrafenib Mesylate structure
|
Common Name | Dabrafenib Mesylate | ||
|---|---|---|---|---|
| CAS Number | 1195768-06-9 | Molecular Weight | 615.668 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C24H24F3N5O5S3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Dabrafenib MesylateDabrafenib Mesylate is a potent and selective Raf kinase inhibitor with IC50s of 0.6 and 5.0 nM for RafV600E and c-Raf, respectively. |
| Name | dabrafenib mesylate |
|---|---|
| Synonym | More Synonyms |
| Description | Dabrafenib Mesylate is a potent and selective Raf kinase inhibitor with IC50s of 0.6 and 5.0 nM for RafV600E and c-Raf, respectively. |
|---|---|
| Related Catalog | |
| Target |
BRafV600E:0.6 nM (IC50) CRAF:5 nM (IC50) |
| In Vitro | Dabrafenib (GSK2118436, 1 μM) with 0.01 μM GSK1120212 inhibits more than 90% of cell growth in the NRAS mutant clones. GSK2118436 is sufficient to reduce S6P phosphorylation in A375[1]. Dabrafenib suppresses the PolyP-mediated vascular barrier permeability, upregulation of inflammatory biomarkers, adhesion/migration of leukocytes, and activation and/or production of nuclear factor-κB, tumor necrosis factor-α, and interleukin-6[2]. Dabrafenib inhibits the release of HMGB1 and downregulates HMGB1-dependent inflammatory responses by enhancing the expressions of cell adhesion molecules (CAMs) in human endothelial cells[3]. |
| In Vivo | Dabrafenib-treated females have mostly immature reproductive tracts with no evidence of ovulation, similar to age-matched controls; however, DAB-treated females have keratinized and histologically open vaginas[5]. |
| Cell Assay | For longer term proliferation assays, cells are plated and treated with compound or combination of compounds in RMPI-1640 containing 10% FBS for 12 days. Compound treatments are replaced at least once during the assay. After 12 days, cells are stained with 0.5% methylene blue in 50% ethanol. Images are captured using flatbed scanner. |
| Animal Admin | The rat pups selected as the test system are derived from 26 10-week-old, time-mated, virus-antibody-free SD (Crl:CD[SD]) female rats. Mated females are observed for natural deliveries from Day 20 to 23 pc (day parturition completed is designated PND 0). Litter examinations are conducted when parturition is complete, on PNDs 3 and 6, and included gender identification, individual pup weights, and external morphologic examinations. Parturient dams and their litters are selected for study based on clinical signs and body weights, and selected dams and their litters are randomized into study groups based on clinical observations and PND 3 litter mean body weights. On PND 3 or 4, litters are culled to four males and five females, with minimal fostering only when necessary to obtain the desired sex ratio, such that natural litters are maintained as much as possible. Records are kept of fostered pups of original and foster dams. All pups are identified by paw tattoo. To the extent possible, nonlittermates are assigned to subsets. DAB is formulated as a suspension in vehicle, 0.5% hydroxypropylmethylcellulose K15M, and 0.1% (v/v) Tween80 in purified water, and is given to juvenile male and female rats orally by gavage at a dose volume of 5 ml/kg, based on daily body weight. |
| References |
| Molecular Formula | C24H24F3N5O5S3 |
|---|---|
| Molecular Weight | 615.668 |
| Exact Mass | 615.089172 |
| PSA | 210.96000 |
| LogP | 7.03360 |
| Storage condition | 2-8℃ |
|
~88% 
Dabrafenib Mesylate CAS#:1195768-06-9 |
| Literature: GLAXOSMITHKLINE LLC; DUMBLE, Melissa; KUMAR, Rakesh; LAQUERRE, Sylvie; LEBOWITZ, Peter Patent: WO2011/47238 A1, 2011 ; Location in patent: Page/Page column 20-21 ; |
|
~% 
Dabrafenib Mesylate CAS#:1195768-06-9 |
| Literature: GLAXOSMITHKLINE LLC; DUMBLE, Melissa; KUMAR, Rakesh; LAQUERRE, Sylvie; LEBOWITZ, Peter Patent: WO2011/47238 A1, 2011 ; |
|
~% 
Dabrafenib Mesylate CAS#:1195768-06-9 |
| Literature: GLAXOSMITHKLINE LLC; DUMBLE, Melissa; KUMAR, Rakesh; LAQUERRE, Sylvie; LEBOWITZ, Peter Patent: WO2011/47238 A1, 2011 ; |
|
~% 
Dabrafenib Mesylate CAS#:1195768-06-9 |
| Literature: GLAXOSMITHKLINE LLC; DUMBLE, Melissa; KUMAR, Rakesh; LAQUERRE, Sylvie; LEBOWITZ, Peter Patent: WO2011/47238 A1, 2011 ; |
|
~% 
Dabrafenib Mesylate CAS#:1195768-06-9 |
| Literature: GLAXOSMITHKLINE LLC; DUMBLE, Melissa; KUMAR, Rakesh; LAQUERRE, Sylvie; LEBOWITZ, Peter Patent: WO2011/47238 A1, 2011 ; |
|
~% 
Dabrafenib Mesylate CAS#:1195768-06-9 |
| Literature: GLAXOSMITHKLINE LLC; DUMBLE, Melissa; KUMAR, Rakesh; LAQUERRE, Sylvie; LEBOWITZ, Peter Patent: WO2011/47238 A1, 2011 ; |
| Benzenesulfonamide, N-[3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluoro-, methanesulfonate (1:1) |
| N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide,methanesulfonic acid |
| Tafinlar |
| N-{3-[5-(2-Amino-4-pyrimidinyl)-2-(2-methyl-2-propanyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide methanesulfonate (1:1) |
| N-[3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide methanesulfonate (1:1) |
| UNII-B6DC89I63E |
| N-{3-[5-(2-aminopyrimidin-4-yl)-2-(1,1-dimethylethyl)thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide monomethanesulfonate |
| dabrafenib mesylate |
| GSK-2118436B |
| Dabrafenib (Mesylate) |
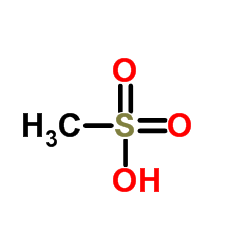
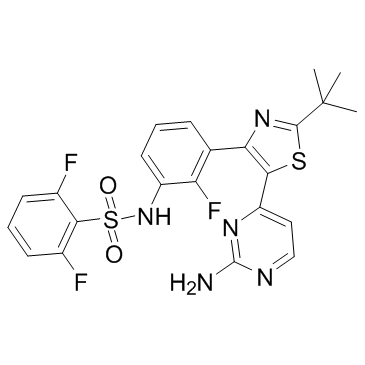
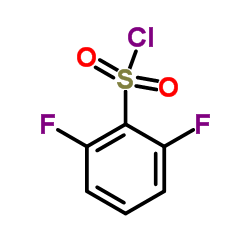
![N-{3-[(2-chloro-4-pyrimidinyl)acetyl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide structure](https://image.chemsrc.com/caspic/004/1195768-20-7.png)
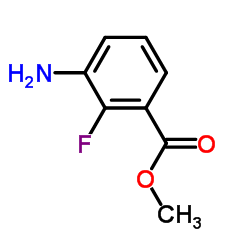
![Methyl 3-{[(2,6-difluoropheyl)sulfonyl]amino}-2-fluorabenzoate structure](https://image.chemsrc.com/caspic/359/1195768-19-4.png)
![N-{3-[5-(2-chloro-4-pyrimidinyl)-2-(1,1-diethylethyl)-1,3-thiazol-4-yl]-2-fluoraphenyl}-2,6-difluorobenzenesulfonamide structure](https://image.chemsrc.com/caspic/380/1195768-23-0.png)