L-CCG-IV
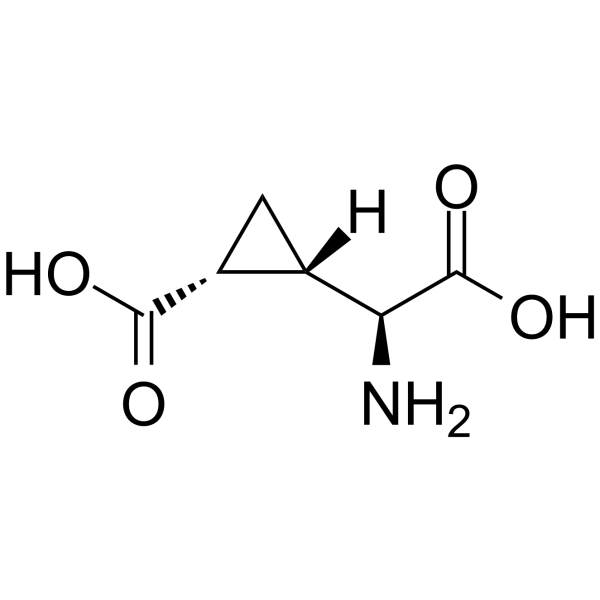
L-CCG-IV structure
|
Common Name | L-CCG-IV | ||
|---|---|---|---|---|
| CAS Number | 117857-95-1 | Molecular Weight | 159.14000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H9NO4 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of L-CCG-IVcis-α-(Carboxycyclopropyl)glycine (L-CCG III) is a potent, competitive glutamate uptake inhibitor. cis-α-(Carboxycyclopropyl)glycine is a substrate of glutamate transporters (GluT) (EC50: 13 μM, 2 μM for EAAT 1 and EAAT 2, respectively). cis-α-(Carboxycyclopropyl)glycine inhibits a Na+-dependent high-affinity L-glutamate uptake in glial plasmalemmal vesicles (GPV) and synaptosomes[1][2]. |
| Name | (1S,2R)-2-[(S)-amino(carboxy)methyl]cyclopropane-1-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | cis-α-(Carboxycyclopropyl)glycine (L-CCG III) is a potent, competitive glutamate uptake inhibitor. cis-α-(Carboxycyclopropyl)glycine is a substrate of glutamate transporters (GluT) (EC50: 13 μM, 2 μM for EAAT 1 and EAAT 2, respectively). cis-α-(Carboxycyclopropyl)glycine inhibits a Na+-dependent high-affinity L-glutamate uptake in glial plasmalemmal vesicles (GPV) and synaptosomes[1][2]. |
|---|---|
| Related Catalog | |
| Target |
glutamate transporters (GluT)[1] |
| References |
| Molecular Formula | C6H9NO4 |
|---|---|
| Molecular Weight | 159.14000 |
| Exact Mass | 159.05300 |
| PSA | 100.62000 |
| Appearance of Characters | solid | off-white |
| Storage condition | Store at RT |
| Water Solubility | H2O: soluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Binding and transport of [3H](2S,4R)- 4-methylglutamate, a new ligand for glutamate transporters, demonstrate labeling of EAAT1 in cultured murine astrocytes.
J. Neurosci. Res. 75(6) , 751-9, (2004) Transporters for L-glutamate (excitatory amino acid transporters; EAATs), localized to astrocytes, are involved intimately in intermediary metabolism within the brain. Because (2S,4R)-4-methylglutamat... |
|
|
Synthesis, molecular modeling studies, and preliminary pharmacological characterization of all possible 2-(2'-sulfonocyclopropyl)glycine stereoisomers as conformationally constrained L-homocysteic acid analogs.
J. Med. Chem. 50 , 4630-41, (2007) Bioisosteric replacements of the distal acidic group of L-glutamic acid (L-Glu, 1) and conformational constraining of its carbon skeleton, have been widely exploited to discover competitive modulators... |
|
|
Involvement of Group II mGluRs in mossy fiber LTD.
Synapse 63(12) , 1060-8, (2009) Mossy fiber long-term depression (LTD) has been shown to be triggered by either pharmacological or synaptic activation of Group II metabotropic glutamate receptors (mGluRs) whereas other studies indic... |
| MFCD00210186 |
| l-ccg-iv |