| Description |
CB7432 is a novel tissue-specific selective estrogen receptor modulator (SERM).
|
| Related Catalog |
|
| Target |
Estrogen receptor[1]
|
| In Vitro |
CB7432 (Idoxifene) possesses the protective roles in vascular smooth muscle cells by its blunting the angiotensin II-induced production of reactive oxygen species (ROS). CB7432 evidently suppresses HSC activation, inhibits culture-activated HSC proliferation in a dose-dependent manner, and induces culture-activated HSC apoptosis in a time-dependent manner[1]. CB7432 (Idoxifene) acts in bone as an estrogen agonist for osteoblasts, and shows negligible agonist activity in human endometrial cells. CB7432 and E2 protect hepatocytes from inflammatory cell injury by inhibiting activation of the NF-κB proinflammatory transcription factor[2].
|
| In Vivo |
Animals receive daily intraperitoneal injections of Estradiol (0.5 mg/kg) and an oral gavage of CB7432 (0.02, 0.1, and 0.5 mg/kg) for 3 days after Dimethylnitrosamine (DMN) treatment. The blood levels of LDH and Estradiol (E2) and histological grades (scores 0 to 5) of liver zone 3 necrosis are evaluated. CB7432 (Idoxifene) at doses of over 0.1 mg/kg significantly reduces the hepatic levels of collagen and MDA in the DMN model in a dose-dependent manner. Although CB7432 and E2 are administered by different routes, i.e., by oral ingestion and intraperitoneal injection, respectively, the antifibrotic effect of a dose of 0.5 mg/kg of CB7432 is somewhat greater than that of the same dose of E2[2].
|
| Cell Assay |
DNA synthesis in cultured HSCs is measured using a Cell Proliferation Biotrack ELISA system. HSCs are cultured in DMEM supplemented with 10% FBS in 96-well plate for 4 d. In the period, the medium is replaced every other day. After 4 d, the culture medium is removed and the same medium with or without 1, 10, or 100 nM of CB7432 (Idoxifene) is added to the cells respectively. After the cells are cultured for an additional 24 h, bromodeoxyuridine (BrdU) is added into each well at a final concentration of 10 μM and the cells are incubated with BrdU for 24 h. The incorporated BrdU is detected[1].
|
| Animal Admin |
Rats[2] Adult male Wistar rats weighing about 200 g are used for the DMN model of hepatic fibrosis. The animals (n=6 in each group), which comprise groups 2 through 6 are administered a single intraperitoneal injection of 40 mg/kg DMN, diluted with saline. The controls in group 1 (n=6) receive a single dose of saline. The rats in groups 4, 5, and 6 receive daily oral gavage of CB7432, in a dosing vehicle (1% methylcellulose) of 10 mL/kg at a dose of 0.02, 0.1, and 0.5 mg/kg/day, respectively, for 14 days after a single injection of DMN. CB7432 is dissolved in vehicle at a dose of 10 mg/mL as a stock solution. The animals in group 3 receive intraperitoneal injections of Estradiol valerate in olive oil at a dose of 0.5 mg/kg/day, for 14 days after the DMN injection. The animals in group 2 receive vehicle only. Animals are anesthetized with 40 mg/kg sodium pentobarbital either 0, 3, 9, or 14 days after DMN injection, and sacrificed. Blood samples are drawn from the inferior vena cava for analyses of serum levels of E2 and lactate dehydrogenase (LDH), a biomarker for necrosis, and liver tissue specimens are taken for light microscopy and immunohistochemistry.
|
| References |
[1]. Zhou YJ, et al. Inhibitory effects of idoxifene on hepatic fibrosis in rats. Acta Pharmacol Sin. 2005 May;26(5):581-6. [2]. Lu G, et al. Antioxidant and antiapoptotic activities of idoxifene and estradiol in hepatic fibrosis in rats. Life Sci. 2004 Jan 2;74(7):897-907.
|
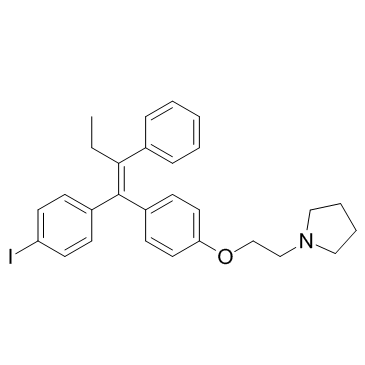
![(E)-1-[4-(2-chloroethoxy)phenyl]-1-(4-iodophenyl)-2-phenyl-1-butene structure](https://image.chemsrc.com/caspic/493/116057-73-9.png)
![1-[4-(2-Chloroethoxy)phenyl]-2-ethyl-2-phenylethanone structure](https://image.chemsrc.com/caspic/196/103628-22-4.png)
![1-[4-(2-chloroethoxy)phenyl]-1-(4-iodophenyl)methanone structure](https://image.chemsrc.com/caspic/297/370563-63-6.png)