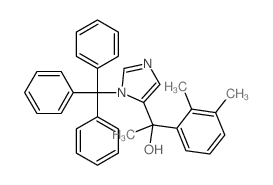
Dexmedetomidine
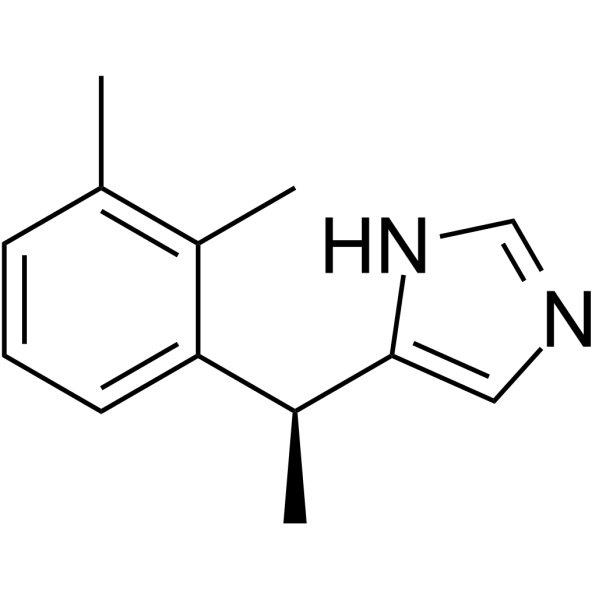
Dexmedetomidine structure
|
Common Name | Dexmedetomidine | ||
|---|---|---|---|---|
| CAS Number | 113775-47-6 | Molecular Weight | 200.28000 | |
| Density | 1.053g/cm3 | Boiling Point | 381.9ºC at 760mmHg | |
| Molecular Formula | C13H16N2 | Melting Point | 146-149°C | |
| MSDS | N/A | Flash Point | 191.3ºC | |
Use of DexmedetomidineDexmedetomidine ((+)-Medetomidine) is a potent, selective and orally active agonist of α2-adrenoceptor, with a Ki of 1.08 nM. Dexmedetomidine shows 1620-fold selectivity against α1-adrenoceptor. Dexmedetomidine exhibits anxiolysis, sedation, and modest analgesia effects[1][2][3]. |
| Name | dexmedetomidine |
|---|---|
| Synonym | More Synonyms |
| Description | Dexmedetomidine ((+)-Medetomidine) is a potent, selective and orally active agonist of α2-adrenoceptor, with a Ki of 1.08 nM. Dexmedetomidine shows 1620-fold selectivity against α1-adrenoceptor. Dexmedetomidine exhibits anxiolysis, sedation, and modest analgesia effects[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
α2-adrenergic receptor:1.08 nM (Ki) |
| In Vitro | Medetomidine has high selectivity for α2 adrenoceptors (Ki=1.08 nM) over α1 adrenoceptors (Ki=1750 nM) in rat brain membranes as measured by the displacement of [3H]clonidine[1]. Medetomidine (0.1-100 nM) inhibits the twitch response in field-stimulated mouse vas deferens, with a pD2 of 9.0[1]. |
| In Vivo | Medetomidine (10-100 μg/kg; i.v. at 5-min intervals) produces a dose-dependent pupillary dilatation in pentobarbitone-anaesthetized rats[1]. Animal Model: Female Sprague-Dawley rats (270-350 g)[1] Dosage: 1, 5, 10, 50, 100 mg/kg Administration: I.v. at 5-min intervals Result: Produced the pupil dilatation of 2.5 mm (approximately half of the maximum effect) at the cumulative dose of 4 μg/kg. |
| References |
| Density | 1.053g/cm3 |
|---|---|
| Boiling Point | 381.9ºC at 760mmHg |
| Melting Point | 146-149°C |
| Molecular Formula | C13H16N2 |
| Molecular Weight | 200.28000 |
| Flash Point | 191.3ºC |
| Exact Mass | 200.13100 |
| PSA | 28.68000 |
| LogP | 3.17830 |
| Vapour Pressure | 1.08E-05mmHg at 25°C |
| Index of Refraction | 1.569 |
| InChIKey | CUHVIMMYOGQXCV-NSHDSACASA-N |
| SMILES | Cc1cccc(C(C)c2cnc[nH]2)c1C |
| Storage condition | 2-8°C |
| HS Code | 2933290090 |
|---|
|
~% 
Dexmedetomidine CAS#:113775-47-6 |
| Literature: NEON LABORATORIES LTD.; DALVI, Mahesh Bhagoji; KENNY, Rajesh Shashikant; TARADE, Pradeep Kisan Patent: WO2013/69025 A1, 2013 ; Location in patent: Page/Page column 7 ; |
|
~% 
Dexmedetomidine CAS#:113775-47-6 |
| Literature: Synthetic Communications, , vol. 26, # 8 p. 1585 - 1593 |
|
~% 
Dexmedetomidine CAS#:113775-47-6 |
| Literature: Synthetic Communications, , vol. 26, # 8 p. 1585 - 1593 |
|
~% 
Dexmedetomidine CAS#:113775-47-6 |
| Literature: Synthetic Communications, , vol. 26, # 8 p. 1585 - 1593 |
|
~% 
Dexmedetomidine CAS#:113775-47-6 |
| Literature: Synthetic Communications, , vol. 26, # 8 p. 1585 - 1593 |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Dexmedetomidine |
| 5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole |
| Dexmedetomidina |
| (S)-medetomidine |
| Dexmedetomidinum |
| 1H-Imidazole,5-[(1S)-1-(2,3-dimethylphenyl)ethyl]- |