Bavisant dihydrochloride hydrate
Modify Date: 2025-08-31 19:32:48
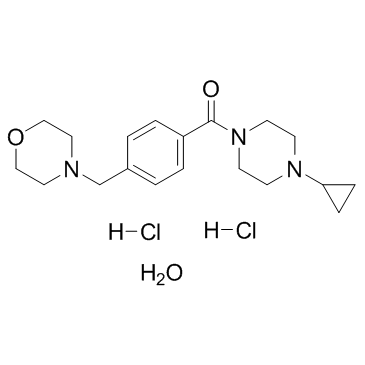
Bavisant dihydrochloride hydrate structure
|
Common Name | Bavisant dihydrochloride hydrate | ||
|---|---|---|---|---|
| CAS Number | 1103522-80-0 | Molecular Weight | 420.37400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C19H31Cl2N3O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Bavisant dihydrochloride hydrateBavisant Hcl hydrate(JNJ-31001074) is a highly selective, orally active antagonist of the human H3 receptor with a novel mechanism of action, involving wakefulness and cognition, with potential as a treatment for ADHD. IC50 Value: Target: H3 receptorin vitro: Bavisant completed a phase II ADHD trial, but no results have been reported [1].in vivo: Mean change from baseline in the total ADHD-RS-IV score at day 42 (primary efficacy endpoint) was -8.8 in the placebo group versus -9.3, -11.2 and -12.2 in the bavisant 1?mg/day, 3?mg/day and 10?mg/day groups, respectively; the change in the 10?mg/day group was not statistically superior to placebo (p=0.161), and hence statistical comparisons of the 1?mg/day and 3?mg/day groups with placebo based on a step-down closed testing procedure were not performed [2].Clinical trial: A Study to Characterize the Pharmacokinetics and Effect of Food on JNJ-31001074 in Healthy Volunteers. Phase 2 |
| Name | (4-cyclopropylpiperazin-1-yl)-[4-(morpholin-4-ylmethyl)phenyl]methanone,hydrate,dihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Bavisant Hcl hydrate(JNJ-31001074) is a highly selective, orally active antagonist of the human H3 receptor with a novel mechanism of action, involving wakefulness and cognition, with potential as a treatment for ADHD. IC50 Value: Target: H3 receptorin vitro: Bavisant completed a phase II ADHD trial, but no results have been reported [1].in vivo: Mean change from baseline in the total ADHD-RS-IV score at day 42 (primary efficacy endpoint) was -8.8 in the placebo group versus -9.3, -11.2 and -12.2 in the bavisant 1?mg/day, 3?mg/day and 10?mg/day groups, respectively; the change in the 10?mg/day group was not statistically superior to placebo (p=0.161), and hence statistical comparisons of the 1?mg/day and 3?mg/day groups with placebo based on a step-down closed testing procedure were not performed [2].Clinical trial: A Study to Characterize the Pharmacokinetics and Effect of Food on JNJ-31001074 in Healthy Volunteers. Phase 2 |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C19H31Cl2N3O3 |
|---|---|
| Molecular Weight | 420.37400 |
| Exact Mass | 419.17400 |
| PSA | 45.25000 |
| LogP | 2.79240 |
| InChIKey | BLFBQJUVAGIUBL-UHFFFAOYSA-N |
| SMILES | Cl.Cl.O.O=C(c1ccc(CN2CCOCC2)cc1)N1CCN(C2CC2)CC1 |
| Storage condition | 2-8℃ |
| Methanone,(4-cyclopropyl-1-piperazinyl)(4-(4-morpholinylmethyl)phenyl)-,hydrochloride,hydrate (1:2:1) |
| (4-Cyclopropylpiperazin-1-yl)(4-(morpholin-4-ylmethyl)phenyl)methanone dihydrochloride monohydrate |
| BAVISANT DIHYDROCHLORIDE |
| Bavisant dihydrochloride (USAN) |
| UNII-C1H7H5X3RE |
| Bavisant dihydrochloride hydrate |
| Bavisant (dihydrochloride hydrate) |