Potassium sulfite
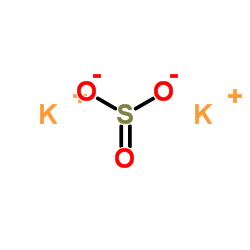
Potassium sulfite structure
|
Common Name | Potassium sulfite | ||
|---|---|---|---|---|
| CAS Number | 10117-38-1 | Molecular Weight | 158.260 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | K2O3S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Name | Potassium Sulfite |
|---|---|
| Synonym | More Synonyms |
| Molecular Formula | K2O3S |
|---|---|
| Molecular Weight | 158.260 |
| Exact Mass | 157.884232 |
| PSA | 82.40000 |
|
Section 1. Chemical Product and Company Identification Potassium sulfite Common Name/ Trade Name Manufacturer Commercial Name(s) Synonym
Chemical Name Chemical Family Potassium sulfite Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Cold water may be used. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product Non-flammable. Auto-Ignition Temperature Not applicable. Flash PointsNot applicable. Flammable LimitsNot applicable. Not available. Products of Combustion Fire Hazards in Presence of Not applicable. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. of Various SubstancesRisks of explosion of the product in presence of static discharge: Not available. Fire Fighting MediaNot applicable. and Instructions Special Remarks onNot available. Fire Hazards Special Remarks on Explosion Not available. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large Spill Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Potassium sulfite Section 7. Handling and Storage PrecautionsDo not breathe dust. Keep away from incompatibles such as oxidizing agents. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Air Sensitive Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearanceSolid. (Powdered solid.Crystalline powder. OdorOdorless. Crystals solid.) TasteNot available. Molecular Weight158.26 g/mole White. Color pH (1% soln/water)Not available. Boiling PointNot available. Melting PointNot available. Critical TemperatureNot available. Not available. Specific Gravity Vapor PressureNot applicable. Not available. Vapor Density VolatilityNot available. Odor ThresholdNot available. Not available. Water/Oil Dist. Coeff. Ionicity (in Water)Not available. See solubility in water. Dispersion Properties SolubilitySoluble in cold water. Section 10. Stability and Reactivity Data StabilityThe product is stable. Not available. Instability Temperature Conditions of InstabilityIncompatible materials, air Incompatibility with variousReactive with oxidizing agents. substancesSlightly reactive to reactive with acids. CorrosivityNon-corrosive in presence of glass. Potassium sulfite Air sensitive. Oxidizes in air to sulfate. Special Remarks on Reactivity Special Remarks onNot available. Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryEye contact. Inhalation. Ingestion. Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans Not available. Other Toxic Effects on Slightly hazardous in case of skin contact (irritant, sensitizer), of ingestion, of inhalation. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onNot available. Chronic Effects on Humans Special Remarks on otherPotential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. May cause contact dermatitis. Eyes: May cause eye irritation. May cause conjunctivitis in sensitive individuals. Inhalation: Can cause respiratory tract irritation. Inhalation can also trigger the onset of hypersensivity reactions, nasal pruritus(itiching) and rhinorrhea (runny nose in sensitized individuals. Ingestion: When ingested it can cause gastric irritation by liberation of sulfurous acid. Because of rapid oxidation to sulfate, sulfites are well tolerated until large doses are reached. Ingestion of large doses can cause nausea, vomiting, abdominal pain, violet colic and diarrhea. It may also cause circulatory disturbances, hypotension (low blood pressure), and may affect behavior/central nervous system (central nervous system stimulation followed by depression, seizures). In animals, hypotension, cyanosis, and cardiovascular collapse have been described following ingestion of lethal doses of sulfites. Some asthmatics are said to be dangerously sensitive to minute amounts of sulfites in foods. Symptoms of hypersensitivity are bronchoconstriction, swelling of the tongue, diffuculty swallowing, diaphoresis (sweating), flushing, urticaria (hives), tachycardia (rapid heart beat), hypotension, and anaphylaxis. Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. Toxicity of the ProductsThe product itself and its products of degradation are not toxic. of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Potassium sulfite Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). IdentificationNot applicable. Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms TSCA 8(b) inventory: Potassium sulfite Federal and State Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No. 233-321-1). Canada: Listed on Canadian Domestic Substance List (DSL). China: Listed on National Inventory. Japan: Listed on National Inventory (ENCS). Korea: Listed on National Inventory (KECI). Philippines: Listed on National Inventory (PICCS). Australia: Listed on AICS. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC) This product is not classified according Not applicable. to the EU regulations. Health Hazard HMIS (U.S.A.)1 National Fire Protection 0 Flammability 0 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) Potassium sulfite ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | Xi: Irritant; |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| HS Code | 2832200000 |
| HS Code | 2832200000 |
|---|
|
Efficient photoelectrochemical hydrogen production from bismuth vanadate-decorated tungsten trioxide helix nanostructures.
Nat. Commun. 5 , 4775, (2014) Tungsten trioxide/bismuth vanadate heterojunction is one of the best pairs for solar water splitting, but its photocurrent densities are insufficient. Here we investigate the advantages of using helic... |
|
|
Allylic selenosulfide rearrangement: a method for chemical ligation to cysteine and other thiols.
J. Am. Chem. Soc. 128 , 2544, (2006) Alkylation of potassium selenosulfate with allylic halides gives Se-allyl seleno Bunte salts. On reaction with thiols at room temperature, these afford mixed dialkyl selenosulfides, which undergo 2,3-... |
| E225 |
| Monopotassium sulfite |
| EINECS 233-321-1 |
| Potassium sulfite |
| potassium sulphite |
| Potassium sulfide |
| Dipotassium sulfite |
| MFCD00011387 |

