209860-87-7
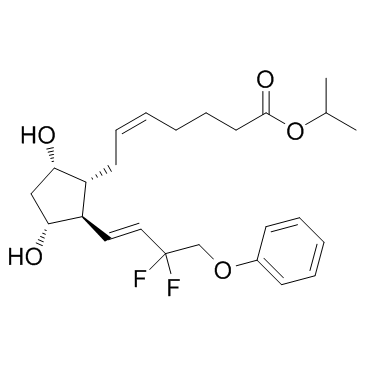
| Name | tafluprost |
|---|---|
| Synonyms |
Tapros
MK-2452 propan-2-yl (Z)-7-[(1R,2R,3R,5S)-2-[(E)-3,3-difluoro-4-phenoxybut-1-enyl]-3,5-dihydroxycyclopentyl]hept-5-enoate Saflutan AFP-168 Isopropyl (5Z)-7-{(1R,2R,3R,5S)-2-((1E)-3,3-difluoro-4-phenoxybut-1-enyl)-3,5-dihydroxycyclopentyl}hept-5-enoate Taflotan Tafluprost Isopropyl (5Z)-7-{(1R,2R,3R,5S)-2-[(1E)-3,3-difluoro-4-phenoxy-1-buten-1-yl]-3,5-dihydroxycyclopentyl}-5-heptenoate UNII-1O6WQ6T7G3 Zioptan |
| Description | Tafluprost(AFP-168) is an anti-glaucoma prostaglandin (PG) analog.Target:OthersTafluprost showed significant IOP-lowering effects without any safety concerns in patients with various types of glaucoma and OH in daily clinical practice and tafluprost is highly effective in any therapeutic patterns [1]. Tafluprost with reduced BAK has potential as a superior antiglaucoma drug, not only for its IOP-lowering effect, but also for its good corneal safety profile [2]. Tafluprost single-use vials treatment was effective in reducing IOP over the 3 years of this study, but visual field performance worsened by 10.3%-13.8% in patients with normal-tension glaucoma. Safety was satisfactory [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 552.9±50.0 °C at 760 mmHg |
| Molecular Formula | C25H34F2O5 |
| Molecular Weight | 452.531 |
| Flash Point | 288.2±30.1 °C |
| Exact Mass | 452.237427 |
| PSA | 75.99000 |
| LogP | 4.23 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.549 |
| Storage condition | -20℃ |