151823-14-2
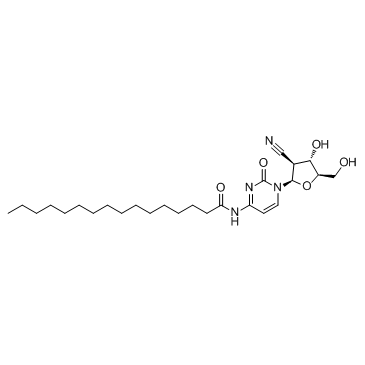
| Name | N-[1-[(2R,3S,4S,5R)-3-cyano-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-2-oxopyrimidin-4-yl]hexadecanamide |
|---|---|
| Synonyms |
1-(2-Cyano-2-deoxy-β-D-arabinofuranosyl)-4-(palmitoylamino)-2(1H)-pyrimidinone
CYC-682 CS-682 Sapacitabine Sapacitabine (CYC682) |
| Description | Sapacitabine is an orally available nucleoside analog prodrug that is structurally related to cytarabine. |
|---|---|
| Related Catalog | |
| Target |
nucleoside analog[1] |
| In Vitro | Concentrations of Sapacitabine required to achieve an IC50 range from 3±0.6 μM for the colon cancer cell line HCT116 to 67±14 μM for the breast cancer cell line MDA-MB-435. Cell cycle analysis shows that 35% Sapacitabine-treated cells are arrested in late-S phase and 41% in G2/M phase. L1210 cells with deoxycytidine kinase (dCK) activity are sensitive to Sapacitabine, (IC50 20±6 μM). In the docetaxel/Sapacitabine combinations, synergistic effects (CI<1) are observed when docetaxel is given before Sapacitabine in both cell lines[1]. |
| In Vivo | On Day 14, the Sapacitabine (5 mg/kg)+vorinostat (33 mg/kg) group has a mean tumour volume of 245 mm3 and a tumour growth inhibition (TGI) of 92%, whereas the Sapacitabine (15 mg/kg)+vorinostat (33 mg/kg) group has a mean tumour volume of 107 mm3 and a TGI of 112%[2]. |
| Cell Assay | A panel of colon (HT29, HCT116, COLO205, HCC2998), breast (MCF7, MDA-MB-435), lung (HOP62, HOP92), ovarian (OVCAR3, IGROV1) cancer cell lines are used in this study. The cell cycle stage and percentage of apoptotic cells are assessed by flow cytometry. In brief, cells are seeded in 25 cm3 flasks and are untreated or treated with various concentrations of Sapacitabine. At the indicated time points, adherent and non-adherent cells are collected, washed with PBS, fixed in 70% ethanol and stored at 4°C until use. Cells are rehydrated in PBS, incubated for 20 min at room temperature (25°C) with 250 μg/mL RNAse A with Triton X-100 and 20 min at 4°C with 50 μg/mL propidium iodide in the dark. The cell cycle distribution and percentage of apoptotic cells are determined with flow cytometer and analysed by FACS Calibur[1]. |
| Animal Admin | Female (nu/nu) mice are injected subcutaneously with 1×107 MV4-11 cells resuspended in 50% Matrigel at a single site on their flanks. Once tumour volumes are 126 to 256 mm3 (16 days post-implantation) animals are pair matched by tumour size into treatment groups (minimum of six mice per group) with a mean tumour size of ~190 mm3. Tumour measurements are calculated using the formula: volume (mm3)=width2 (mm)×length (mm)×0.5. Sapacitabine is administered once a day orally (5 or 15 mg/kg) for 4 days, followed by a 3-day break before another 4 days of treatment; dosing starts on the same day as distribution to the treatment groups[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C26H42N4O5 |
| Molecular Weight | 490.635 |
| Exact Mass | 490.315521 |
| PSA | 137.47000 |
| LogP | 5.67 |
| Index of Refraction | 1.575 |
| Storage condition | 2-8℃ |